| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Case Report
Volume 3, Number 4, December 2014, pages 154-156
Imaging Characteristics of 2,8-Dihydroxyadenine Stones: Review of a Rare Disorder
Ethan B Frama, c, Robert G Berkenblita, David M Hoenigb
aAlbert Einstein College of Medicine, Bronx, NY, USA
bLong Island Jewish Medical Center, New Hyde Park, NY, USA
cCorresponding Author: Ethan B Fram, 1945 Eastchester Rd, 14A, Bronx, NY 10461, USA
Manuscript accepted for publication May 08, 2014
Short title: 2,8-Dihydroxyadenine Stones
doi: http://dx.doi.org/10.14740/wjnu160w
| Abstract | ▴Top |
Adenine phosphoribosyl transferase (APRT) deficiency is a rare genetic condition that often presents with recurrent dihydroxyadenine (DHA) urolithiasis. To our knowledge, no CT imaging of DHA stones has been previously reported in English medical literature. We report a case of an 86-year-old woman with a large DHA stone in her upper left ureter. On CT imaging the stone was low density and had an unusual linear central lucency. We also review the literature for this rare disorder.
Keywords: CT; 2,8-dihydroxyadenine; Adenine phosphoribosyl transferase deficiency; Urolithiasis
| Introduction | ▴Top |
Formation of 2,8-dihydroxyadenine (DHA) stones is a rare form of urolithiasis that occurs in patients with adenine phosphoribosyl transferase (APRT) deficiency. Standard chemical and thermogravimetric analysis does not differentiate DHA stones from uric acid or xanthine stones, and either infrared or ultraviolet spectroscopy is required to make the diagnosis [1]. DHA stones are typically, but not always, radiolucent [2]. We report a case of 2,8-DHA urolithiasis, treated by percutaneous nephrostolithotomy (PCNL). To our knowledge, CT imaging characteristics of a DHA stone have not been previously reported in English medical literature.
| Case Report | ▴Top |
We report on an 87-year-old female who presented with recurrent, severe flank pain. Non-contrast CT revealed a 1.7 × 1.4 cm smooth, ovoid calculus in the proximal left ureter (Fig. 1). This calculus measured 272 Hounsfield units and had a linear low density center. The 5 mm thick images from a 64 slice multidetector CT scanner (CTi, General Electric Medical Systems, Milwaukee, WI) were obtained. Scanning parameters were at 120 kVp and 240 mA; 0.625 mm source images were obtained and reconstructed to 5 mm thick images for review at a PACS station. A dipstick urinalysis the 1 day prior to surgery was unremarkable, with a pH of 7. The patient underwent left PCNL for removal of the stone. During surgery, the stone was noted to be white yellow in coloration, soft and easily fragmented, and with a lamellar internal appearance. Stone analysis using infrared spectroscopy indicated that the stone was pure 2,8-DHA. The patient was discharged and has not reported further incidence of stones in 7-year follow-up.
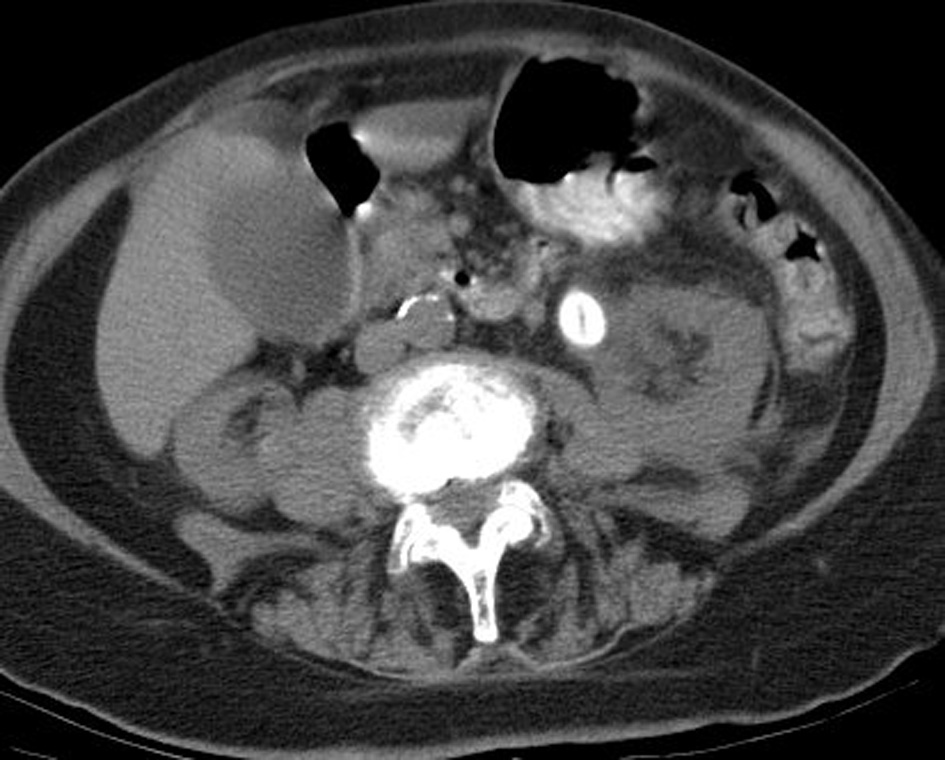 Figure 1. CT image showing calculus. Figure 1. CT image showing calculus. |
| Discussion | ▴Top |
CT imaging
The calculus was in the lower range of density for uric acid stones (386 ± 154 HU) [3] and CT imaging by itself was not sufficient for discrimination between the two. It can be expected that most DHA stones will be radiolucent on plain abdominal radiographs, based both on previous reports and on the low attenuation of this stone [4]. The linear central lucency may be characteristic of DHA stones or an unusual development in this patient, but may represent a significant means of differentiation from other low density stones if observed in other patients. To be certain, additional reports of CT imaging of DHA stones are needed.
Etiology of DHA stones
The 2,8-DHA is the end product of adenine metabolism by xanthine oxidase in the absence of APRT (Fig. 2). Preferential excretion of DHA and low solubility in urine promote crystallization [5]. The solubility of DHA in urine is not strongly affected by pH. Over an 8-year period patient A had an average urinalysis pH of 6.6 (11 readings) and no reading lower than 6.0. A uric acid seeming stone formed with no urine pH measurements < 6 should be suspect for DHA.
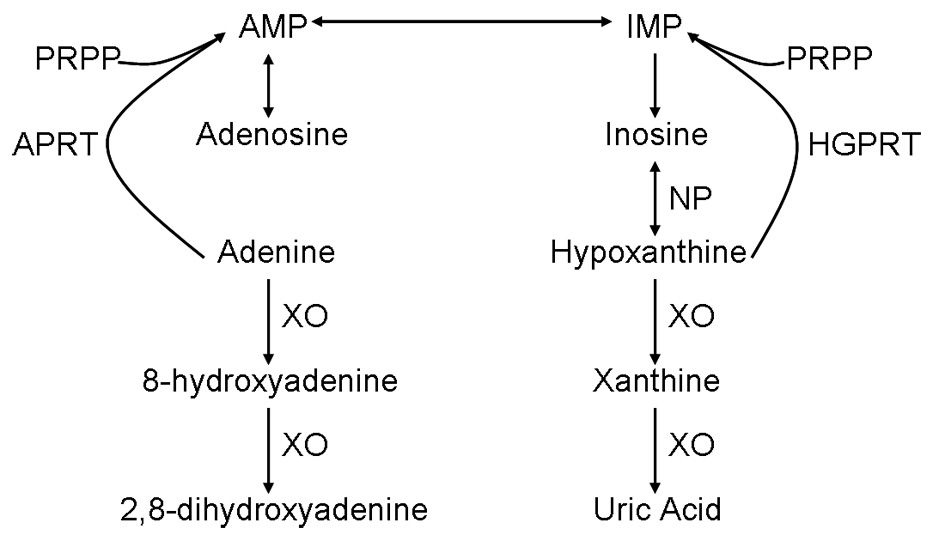 Figure 2. Normal adenine metabolism. Figure 2. Normal adenine metabolism. |
Prevalence and recognition
APRT deficiency is an underdiagnosed disorder in the United States. With an estimated prevalence of 1/50,000 - 1/100,000 [6] and less than 300 reported cases worldwide, most individuals with APRT deficiency are unaware of their condition. This is due to both a lack of awareness of the disorder among physicians and the challenges of differentiating DHA stones from other purine stones.
Risks of APRT deficiency
Identifying APRT deficiency is important due to the long-term risks associated with the condition. Approximately 90% of patients with APRT deficiency experience urolithiasis, often beginning at a young age [7]. A significant proportion of these patients will develop chronic kidney disease which may progress to end-stage renal disease (ESRD). For APRT patients, with or without urolithiasis, there is a risk of developing ESRD due to crystalline nephropathy. Because the kidney failure is secondary to a systemic disorder, transplanted kidneys will also fail unless the disease is recognized and treated [8]. No other organ systems have been reported to be affected by APRT deficiency.
Identification of APRT deficiency
Bollee et al discuss the identification of 53 cases of APRT deficiency, finding that stone analysis was the most common method, followed by crystalluria. The 2,8-DHA stones are variable in color, with reports from reddish brown to grey and whitish yellow to grey blue [9]. If APRT deficiency is suspected, stones should be analyzed using infrared spectroscopy or ultraviolet spectroscopy at pH 2 and 10, as alkaline ultraviolet spectroscopy will not differentiate DHA stones from uric acid stones. On polarized light microscopy, DHA crystals appear round and reddish brown, with a characteristic central Maltese cross pattern [10]. Confirmation of APRT deficiency can be performed by assaying for APRT activity in red cell lysate or through genetic testing in populations in which the deficiency has been characterized [11].
Treatment and outcomes
The medical treatment for APRT deficiency centers on the use of allopurinol or febuxostat to inhibit the conversion of adenine to 2,8-DHA [12]. Adenine is readily excreted and does not appear to contribute to stone formation. Edvardsson et al (2001) observed an allopurinol dosage of 5 - 10 mg/kg/day to be effective at preventing most recurrences of DHA stones. As the solubility of DHA is not significantly altered by pH, a high fluid intake without alkalinization is recommended. A low purine diet, as for recurrent uric acid urolithiasis, is also beneficial. APRT deficiency can be controlled, but early detection to prevent kidney damage is critical.
Conflict of Interest
The authors have no conflicts of interest.
| References | ▴Top |
- Marra G, Vercelloni PG, Edefonti A, Manzoni G, Pavesi MA, Fogazzi GB, Garigali G, et al. Adenine phosphoribosyltransferase deficiency: an underdiagnosed cause of lithiasis and renal failure. JIMD Rep. 2012;5:45-48.
doi pubmed - Yagisawa T, Yamazaki Y, Toma H, Kamatani N. Radiopaque 2,8-dihydroxyadenine lithiasis. Int Urol Nephrol. 1999;31(2):141-143.
doi pubmed - Bellin MF, Renard-Penna R, Conort P, Bissery A, Meric JB, Daudon M, Mallet A, et al. Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. Eur Radiol. 2004;14(11):2134-2140.
doi pubmed - Chua ME, Gatchalian GT, Corsino MV, Reyes BB. Diagnostic utility of attenuation measurement (Hounsfield units) in computed tomography stonogram in predicting the radio-opacity of urinary calculi in plain abdominal radiographs. Int Urol Nephrol. 2012;44(5):1349-1355.
doi pubmed - Sahota AS, Tischfield JA, Kamatani N, Simmonds HA. Adenine Phosphoribosyltransferase Deficiency and 2,8-Dihydroxyadenine Lithiasis. Valle D, Beaudet AL, Vogelstein B, Kinzler KW, et al, eds. Online Metabolic and Molecular Bases of Inherited Disease. http://www.ommbid.com. Published January 2006. Updated March 28, 2011. Accessed June 19, 2013.
- Edvardsson VO, Palsson R, Sahota A. Adenine Phosphoribosyltransferase Deficiency. 2012 Aug 30. In: Pagon RA, Adam MP, Bird TD, et al., editors. GeneReviews? [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK100238/.
- Bollee G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, Deteix P, et al. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21(4):679-688.
doi pubmed - Nasr SH, Sethi S, Cornell LD, Milliner DS, Boelkins M, Broviac J, Fidler ME. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transplant. 2010;25(6):1909-1915.
doi pubmed - Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T. Clinical features and genotype of adenine phosphoribosyltransferase deficiency in iceland. Am J Kidney Dis. 2001;38(3):473-480.
doi pubmed - Harambat J, Bollee G, Daudon M, Ceballos-Picot I, Bensman A. Adenine phosphoribosyltransferase deficiency in children. Pediatr Nephrol. 2012;27(4):571-579.
doi pubmed - Cassidy MJ, McCulloch T, Fairbanks LD, Simmonds HA. Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient. Nephrol Dial Transplant. 2004;19(3):736-738.
doi pubmed - Simmonds HA, Van Acker KJ, Cameron JS, Snedden W. The identification of 2,8-dihydroxyadenine, a new component of urinary stones. Biochem J. 1976;157(2):485-487.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
