| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Case Report
Volume 3, Number 3, September 2014, pages 129-133
Plasma Cell Leukemia Presenting as Acute Kidney Injury With Heavy Proteinuria
Varsha Podduturia, Hashim Mohmandb, Javed Gilla, Xin J. Zhoua, c, d
aDepartment of Pathology, Baylor University Medical Center, Dallas, TX, USA
bDallas Nephrology Associates, Dallas, TX, USA
cRenal Path Diagnostics, Pathologists BioMedical Laboratories, Lewisville, TX, USA
dCorresponding Author: Xin J. Zhou, Renal Path Diagnostics, Pathologists BioMedical Laboratories, 2501 S. State Highway 121, Suite 1220, Lewisville, TX 75067, USA
Manuscript accepted for publication July 31, 2014
Short title: Plasma Cell Leukemia
doi: https://doi.org/10.14740/wjnu177w
| Abstract | ▴Top |
Plasma cell leukemia (PCL) is a rare and aggressive malignancy of plasma cells with a poor prognosis. Direct renal involvement in PCL is rarely reported with only a few cases in the English literature. We described the clinical and pathologic findings of a 40-year-old African-American male with acute kidney injury (AKI) due to light-chain cast nephropathy and direct renal invasion by malignant plasma cells, diagnosed as having PCL on renal biopsy and successfully treated with aggressive plasmapharesis and allogeneic stem cell transplantation (SCT). To the best of our knowledge, this is the first case report of PCL diagnosed on kidney biopsy. Among the various clinical manifestations of PCL, AKI and proteinuria may be its initial presentation, illustrating the etiologic complexity of AKI. Successful treatment of cast nephropathy with plasma exchange and PCL by SCT was associated with recovery of renal function.
Keywords: Plasma cell leukemia; Acute renal failure; Renal pathology
| Introduction | ▴Top |
Plasma cell leukemia (PCL) is an uncommon and aggressive plasma cell dyscrasia and is diagnosed when peripheral blood absolute plasma cell count exceeds 2.0 × 109/L or circulating plasma cells are greater than 20% [1]. PCL is either primary or after a prodrome of multiple myeloma (MM). Both primary and secondary PCL occur in the fifth and sixth decades with a male predominance. PCL accounts for 0.2% of all leukemias diagnosed in 1997-2000 [2]. Signs and symptoms are similar to those seen in MM such as thrombocytopenia, anemia, renal insufficiency, hypercalcemia, lytic bone lesions, and can also include hepatomegaly and splenomegaly [3]. We report a case of a 40-year-old man who presented with acute renal failure and heavy proteinuria and was found to have primary PCL invading the kidney coupled with light-chain cast nephropathy who has been successfully treated with stem cell transplantation (SCT).
| Case Report | ▴Top |
A 40-year-old African-American male with hypertension and chronic back pain presented to the emergency department with abdominal pain, nausea, vomiting, and back pain. Body weight was 280 pounds and blood pressure was 157/100 mm Hg. There was no pedal edema. Laboratory data showed white blood cell count 15.1 × 103/µL, hemoglobin 9.5 g/dL, hematocrit 29.2%, platelet count 143 × 109/L, BUN 39 mg/dL, creatinine 5.1 mg/dL, and calcium 10.2 mg/dL. Initial urinalysis showed 2+ blood, 2+ protein, and 2+ leukocyte esterase. He was admitted and repeat urinalysis the next day was dipstick negative for protein. However, 24-h urinary protein was 16,559 mg. Serum protein electrophoresis demonstrated two paraprotein bands in the gamma region. Band 1 was 0.5 g/dL and band 2 was 0.52 g/dL. Free kappa light chain measured 0.89 mg/dL and lambda light chain > 1,500 mg/dL (normal range 0.57 - 2.63 mg/dL). Antinuclear antibody screen, ANCA and anti-glomerular basement membrane antibodies were negative. Ultrasound revealed large echogenic kidneys bilaterally measuring 13.1 cm and 13.0 cm on the right and left sides, respectively. He underwent a percutaneous renal biopsy for further evaluation.
Renal biopsy showed two small fragments of renal cortex and medulla with seven histologically unremarkable glomeruli present; none was globally sclerotic (Fig. 1). There was mild tubular atrophy and interstitial fibrosis involving 25% of the renal cortex with clusters of dense interstitial infiltrates composed of atypical plasma cells (Fig. 1). Occasional tubules contain dense casts surrounded by cellular response (Fig. 1). Immunofluorescence studies showed negative glomerular staining for IgG, IgA, IgM, C1q, C3, albumin, fibrinogen, kappa and lambda light chains. Several casts and tubular cytoplasmic protein droplets stained for lambda light chain (4+). Interstitial plasma cells stained for lambda light chain (2+). Kappa light chain was negative. Plasma cells were immunohistochemically reactive for CD138 (Fig. 1). Electron microscopy (EM) revealed no electron dense deposits. Glomerular basement membranes were unremarkable with approximately 10% podocyte foot process effacement (Fig. 1).
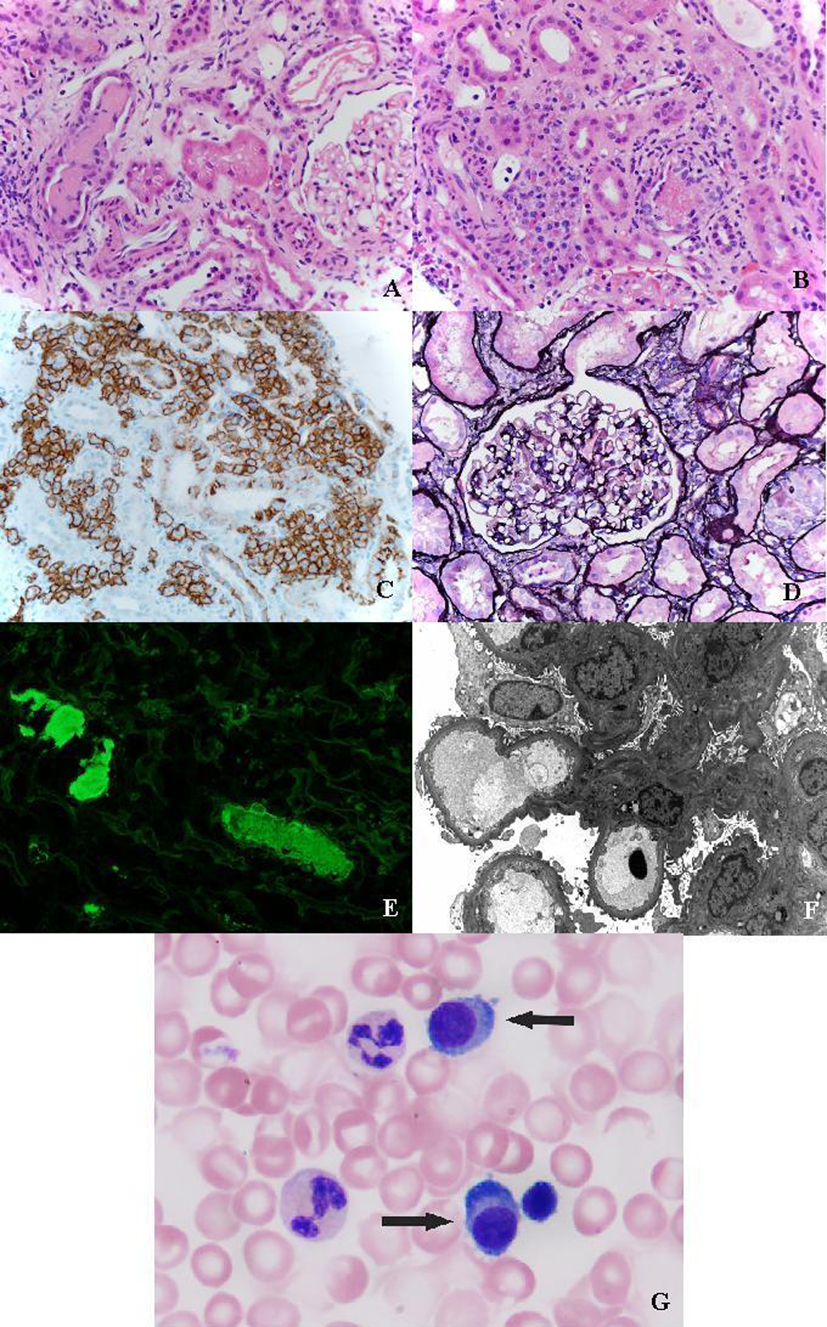 Click for large image | Figure 1. (A) Light-chain cast surronunded by giant cells (H&E, × 400). (B) Interstitial infiltration of leukemic plasma cells (H&E, × 400). (C) Immunohistochemical staining for CD138 highlighting plasma cells. (D) Silver stain showing normal glomeruli (× 400). (E) Tubular casts strongly positive for lambda chain (immunofluorescence × 400). (F) Electron micrograph showing unremarkable glomerulus. (G) Plasma cells in peripheral blood (Wright stain, × 100). |
Bone marrow biopsy and aspirate were performed. Peripheral blood smear revealed 23% plasma cells (absolute plasma cell count at 3.4 × 109) (Fig. 1). Bone marrow biopsy showed mostly cortical bone with small groups of plasma cells. The aspirate consisted of mostly blood. Flow cytometry demonstrated 25% population of plasma cells expressing CD38, CD56, and monoclonal restriction of intracytoplasmic lambda light chain. The final diagnosis of PCL involving the kidney with lambda light-chain cast nephropathy was made.
MRI of the spine revealed compression fractures of T10, T12, L2 and L4 without central canal impingement. Vertebral biopsies showed hypercellular (up to 80%) bone marrow with increased numbers of immature plasmacytoid forms. ISH studies for kappa and lambda light chains revealed a marked lambda light-chain restriction (> 100:1). He was started on daily plasmapheresis and chemotherapy (Bortezomib and Lenalidomide) for nine consecutive days during which there was a rapid reduction in free light-chain levels and serum creatinine. At the end of his stay, free kappa light chain measured 0.04 mg/dL, lambda light chain 121.00 mg/dL and creatinine 3.3 mg/dL. His chemotherapy regimen continued as an outpatient. Three months later, the patient was hospitalized for an HLA and ABO identical and sex-matched allogeneic stem cell transplant from his sibling. At the end of his hospital course for the stem cell transplant, the creatinine measured 1.0 mg/dL. At a 24-month clinic follow-up (post-SCT), he remains free of residual or recurrent disease with normalized renal function.
| Discussion | ▴Top |
Acute kidney injury (AKI) has various etiologies and is divided into pre-renal, renal, and post-renal causes [4]. Renal biopsy plays a major role in the diagnosis and management of AKI [5]. In the current case, the presence of AKI coupled with heavy proteinuria and free lambda chain on urine protein electrophoresis led to a primary consideration of amyloidosis or light-chain deposition disease in the clinical differential diagnosis. Surprisingly, the biopsy revealed kidney infiltration by PCL coupled with light-chain cast nephropathy. Although 24-h urine collection showed > 16 g of protein, the urine dipstick yielded a negative result. This discrepancy can be explained by the fact that urine dipstick test is sensitive to albumin but not to globulins or Bence-Jones proteins because of the increased number of free amino groups in the latter. Thus, the protein in urine represented freely filtered lambda light chain rather than albumin.
PCL is an uncommon and aggressive malignancy. Treatment includes induction therapy with alkylating agents. Other therapies include autologous and allogeneic SCT. Several cases of primary PCL treated with SCT have been reported, with a majority receiving autologous stem cell transplants. Few cases of allogeneic SCT have been reported, therefore making it difficult to compare survival rates with autologous SCT. The average age of these patients was 54 years and survival averages 28 months and ranges from 7 to 106 months [6]. The overall 5-year survival rate from the time of diagnosis is less than 10% [7].
Only a few cases of PCL involving kidney have been previously reported (Table 1). Rahman and colleagues reported a 45-year-old woman who presented with nausea and vomiting and 39% plasma cells were found in peripheral blood [8]. The urine revealed no monoclonal free light chains. Bone marrow aspirate showed sheets of plasma cells. Initial tests showed BUN 41 mg/dL and creatinine 2.5 mg/dL. Renal biopsy revealed plasma cells in the interstitium and tubular casts. Immunofluorescence microscopy (IF) showed a 4+ linear distribution of kappa light chains along the tubular and glomerular basement membranes. Aggregates of granular and fibrillar material were present in the mesangium, glomerular basement membrane and preglomerular arterial wall on EM. The final diagnosis was PCL and kappa light-chain deposition disease. The patient began chemotherapy and achieved remission. Creatinine measured 1.7 mg/dL at the end of chemotherapy [8]. No clinical follow-up were performed.
 Click to view | Table 1. Clinical Spectrum of Plasma Cell Leukemia Involving Kidney |
A second case [9] described a 50-year-old male who presented with vomiting, diarrhea, and oliguria for 2 days. Lab values included hemoglobin 5.8 g/dL, platelets 31 × 109/L, and 39% plasma cells in peripheral blood. Bone marrow aspirate showed 77% plasma cells. Multiple osteolytic lesions were discovered in the X-ray of the skull. Serum creatinine measured 8.3 mg/dL and BUN was 190 mg/dL. After the diagnosis of primary PCL was made, the patient received cyclophosphamide, prednisolone, and dexamethasone. No renal biopsy was performed and the patient was lost to follow-up.
A third case [10] described a 52-year-old woman presenting with nausea, vomiting, and dizziness. Labs showed platelets 70 × 109/L, hematocrit 20%, and serum creatinine 3.69 mg/dL. Peripheral blood smear showed 30% plasma cells. Bone marrow biopsy demonstrated 60-70% plasma cells. Serum protein electrophoresis showed an IgG lambda monoclonal protein. No lytic lesions were identified on bone survey. Treatment with high-dose dexamethasone and thalidomide began and serum creatinine improved to 2 mg/dL. Although therapy was continued for the next 2 months, serum creatinine rose to 3.8 mg/dL. Renal biopsy showed an interstitial infiltrate of atypical plasma cells that were strongly lambda positive by in situ hybridization. A diagnosis of PCL involving kidney was made. On IF, the glomeruli demonstrated no staining for IgG, IgA, IgM, Clq, C3, fibrin, kappa and lambda light chains. EM failed to show glomerular deposits. Despite radiation to the kidney and cyclophosphamide, renal function remained the same and the patient remained on dialysis.
In summary, this is the first case report of PCL diagnosed on kidney biopsy and successfully treated by allogeneic SCT. This case serves as a reminder that the etiologies of AKI are complex and prompt recognition and diagnosis on the basis of clinical and pathological findings with appropriate therapy is critical in the disease management.
Disclosures
We report no conflicts of interest or support/funding with this manuscript.
| References | ▴Top |
- Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, Thiele J, Vardiman J (Eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC; 2008:203-204.
- Herrera GA. Renal lesions associated with plasma cell dyscrasias: practical approach to diagnosis, new concepts, and challenges. Arch Pathol Lab Med. 2009;133(2):249-267.
pubmed - Albarracin F, Fonseca R. Plasma cell leukemia. Blood Rev. 2011;25(3):107-112.
doi pubmed - Jones M. Recognising acute kidney injury. Clin Med. 2012;12(3):287-289.
doi pubmed - Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756-766.
doi - Saccaro S, Fonseca R, Veillon DM, Cotelingam J, Nordberg ML, Bredeson C, Glass J, et al. Primary plasma cell leukemia: report of 17 new cases treated with autologous or allogeneic stem-cell transplantation and review of the literature. Am J Hematol. 2005;78(4):288-294.
doi pubmed - Fernandez de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, Hajek R, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27(4):780-791.
doi pubmed - Rahman A, Mossey RT, Susin M, Budman D, Mailloux LU. Kappa-chain nephropathy associated with plasma cell leukemia. Arch Intern Med. 1984;144(8):1689-1691.
doi pubmed - Kalyani R, Kumar ML. Plasma cell leukemia presenting as acute renal failure: a case report. Indian J Pathol Microbiol. 2007;50(1):86-88.
pubmed - Lommatzsch SE, Bellizzi AM, Cathro HP, Rosner MH. Acute renal failure caused by renal infiltration by hematolymphoid malignancy. Ann Diagn Pathol. 2006;10(4):230-234.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
