| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Case Report
Volume 4, Number 3, September 2015, pages 247-250
Intermittent Dialysis Leading to Dialysis Disequilibrium Syndrome
Kalyani Regetia, Waqas Jehangira, c, Sherry Kumarb, Chadrick Chuaa, Abdalla Yousifa, Shuvendu Sena
aRaritan Bay Medical Center, Perth Amboy, NJ 08861, USA
bRoss University School of Medicine, Miramar, FL, USA
cCorresponding Author: Waqas Jehangir, Raritan Bay Medical Center, 530 New Brunswick Ave, Perth Amboy, NJ 08861, USA
Manuscript accepted for publication July 15, 2015
Short title: Dialysis Disequilibrium Syndrome
doi: http://dx.doi.org/10.14740/wjnu228e
| Abstract | ▴Top |
Dialysis disequilibrium syndrome (DDS) is a critical and ultimately fatal condition that presents in hemodialysis patients. Serious manifestations of DDS involve impaired concentration, disorientation, and coma. Risk factors for the condition include dialysis treatment, elevated BUN, renal disease, metabolic acidosis, and pre-existing neurologic disease. There are three main theories proposed to account for the development of DDS, including a reverse urea effect, idiogenic osmoles, and paradoxical brain acidosis. Each of these theories potentiates cerebral edema, and eventually brain herniation, that leads to death. This case examines a 33-year-old Hispanic male brought to the emergency room. He was admitted to the ICU based on hospital findings and had a metabolic acidosis that persisted despite appropriate initial treatment. He was eventually placed on hemodialysis over the course of 2 weeks, and ultimately expired from DDS. This paper aims to demonstrate that hemofiltration is a superior alternative in patients where hemodialysis is indicated, so that DDS, and subsequent fatality, can be avoided.
Keywords: Dialysis disequilibrium syndrome; Herniation; Hemodialysis
| Introduction | ▴Top |
Dialysis disequilibrium syndrome (DDS) is a rare and serious complication of hemodialysis that manifests with neurological symptoms including impaired concentration, hallucinations, disorientation, and coma, if left untreated. These neurological symptoms are primarily attributed to cerebral edema [1]. Furthermore, brain death can eventually ensue and become fatal. DDS can present after receiving first time dialysis treatment, or intermittent dialysis. First time hemodialysis patients, especially those with markedly elevated blood urea nitrogen levels, are at greatest risk for DDS [1].
| Case Report | ▴Top |
A 33-year-old Hispanic male was brought to the hospital by emergency services after he was found unconscious at home. At the time, the patient’s vitals included heart rate of 74/min, respiratory rate of 26/min, and a systolic blood pressure of 80 mm Hg. His pupils were round and reactive to light. The remainder of his physical examination was unremarkable.
Initial laboratory investigations revealed a white blood cell count of 24.7 × 103/mm3, blood urea nitrogen of 17 mg/dL, serum creatinine of 3.5 mg/dL, anion gap of 27, arterial blood gases pH of 6.95, PaCO2 79 mm Hg, PaO2 < 37 mm Hg, HCO3 17.4 mmol/L, and lactate 24 mmol/L. These findings were consistent with increased leukocytosis, high anion gap metabolic acidosis, and respiratory acidosis. The metabolic acidosis was partially accounted for by acute renal failure with retained unmeasured anions and ketonemia. Liver function tests further demonstrated elevated ALT and AST. Creatinine kinase and CKMB were also found to be elevated. Toxicology reports and urine drug screen were positive for cocaine and opiates. Urinalysis was negative for infection causing organisms. Electrocardiogram showed normal sinus bradycardia (Fig. 1). Computed tomography (CT) of the head was negative for intracranial bleed and did not show any anoxic encephalopathy (Fig. 2). The patient had intact gag and cough reflexes. Based on laboratory and radiographic findings, the patient was admitted to the ICU with a provisional diagnosis of cardiopulmonary arrest secondary to drug overdose, acute kidney failure, and septic shock. The patient was empirically started on vancomycin and piperacillin and tazobactam.
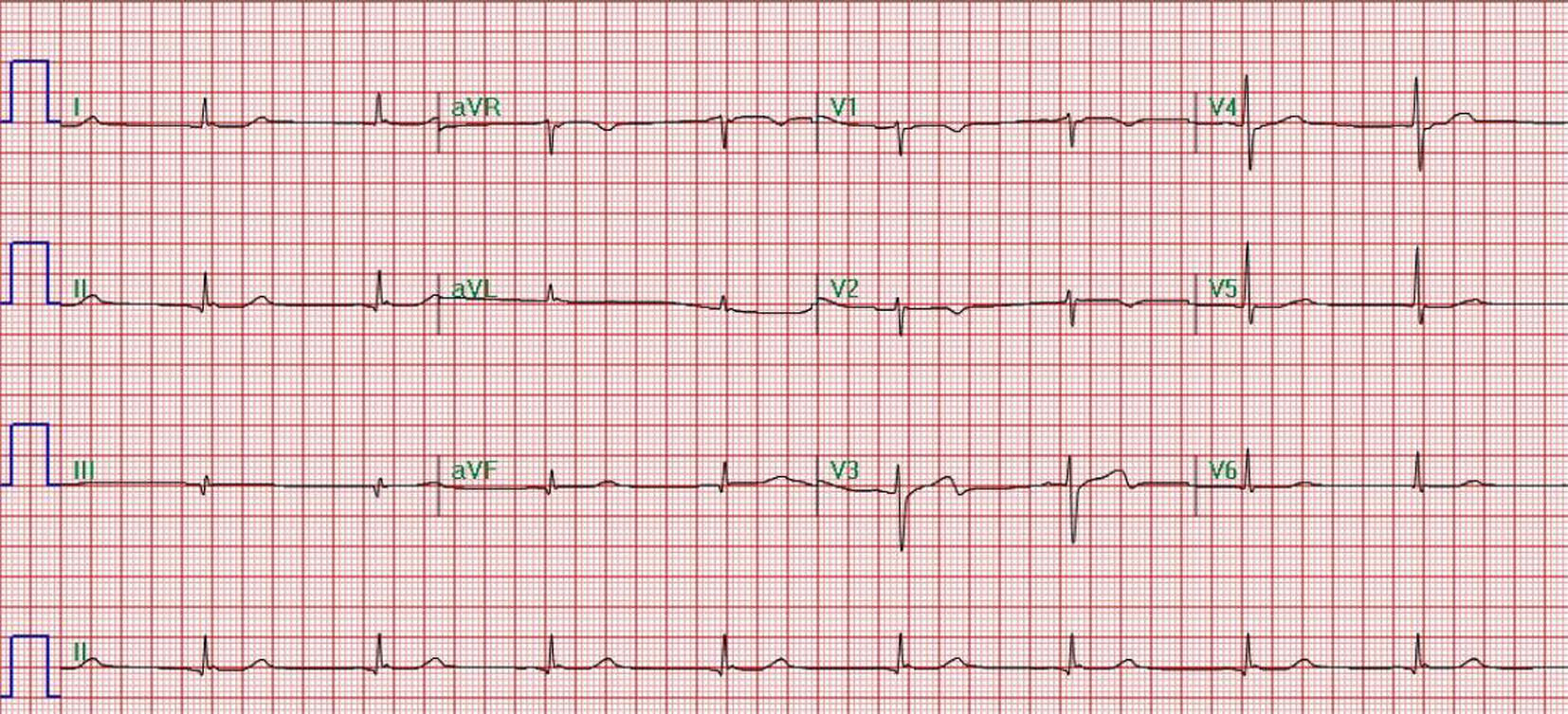 Click for large image | Figure 1. Normal sinus bradycardia. |
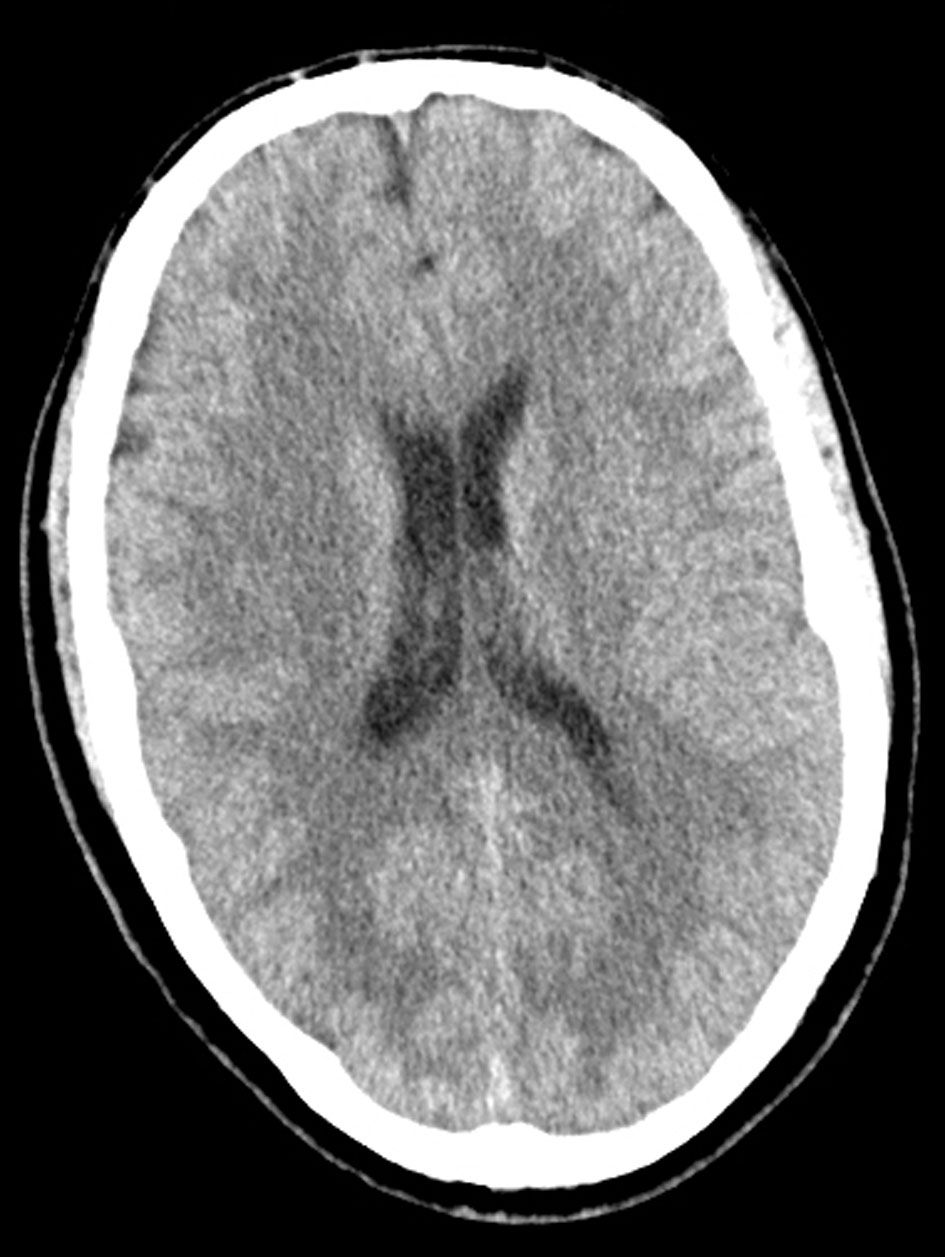 Click for large image | Figure 2. Computed tomography (CT) of the head was negative for intracranial bleed and did not show any anoxic encephalopathy. |
Despite being placed on a sodium bicarbonate drip (150 mEq of 8.4% sodium bicarbonate in 1000 D5W), his metabolic acidosis persisted. He was further given fluid resuscitation of about 3 L, and still remained oliguric. A decision was made by the patient’s nephrologist to start hemodialysis. The sputum culture showed many Streptococcus serogroup C and urine culture showed Klebsiella pnuemoniae ESBL. During a hospital stay of approximately 14 days, the patient received dialysis three times per week. However, his blood urea nitrogen and serum creatinine levels still failed to improve, and he remained in a state of metabolic acidosis. On day 14 of his admission, the patient’s condition deteriorated 2 h in dialysis treatment. He lost his gag and cough reflexes, and his pupils became dilated and fixed. A repeat head CT scan was performed which showed evidence of brain herniation. The brain herniation was confirmed using brain scan flow which showed empty bulb sign (Fig. 3). Repeat lab investigations conducted immediately following hemodialysis revealed a pH of 7.24, HCO3 of 26, sodium of 134 mmol/L, potassium 3.0 mmol/L, urea of 89 mg/dL, and serum creatinine of 11.2 mg/dL. Ultimately, the patient was pronounced brain dead the patient’s family opted for organ donation.
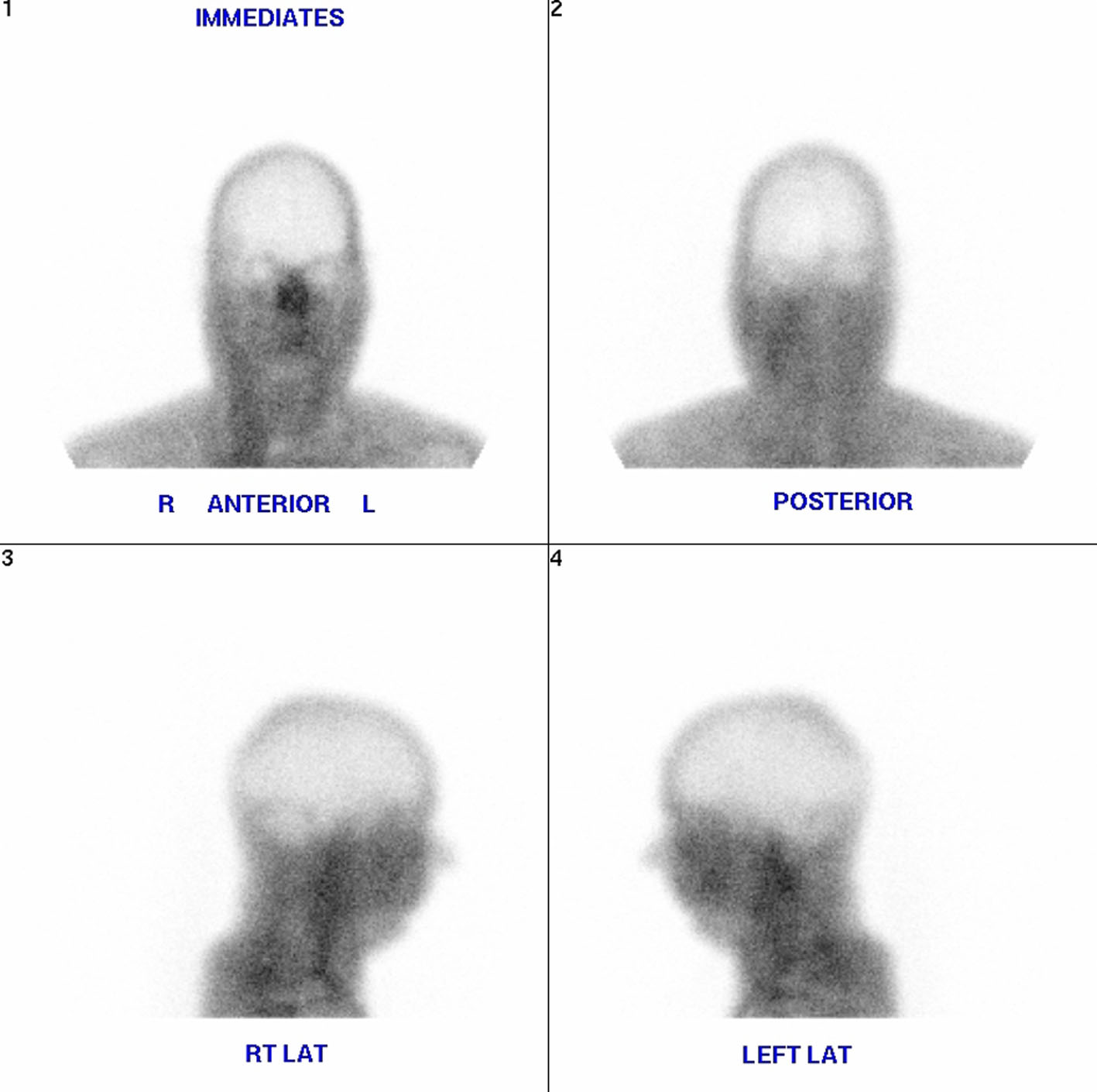 Click for large image | Figure 3. Brain scan flow which showed empty bulb sign. |
| Discussion | ▴Top |
DDS is a rare, but serious disease, with fatal complications including cerebral edema and brain death. DDS occurs during or after dialysis [1]. Risk factors for DDS include dialysis treatment, markedly elevated blood urea concentration, predialysis (i.e. > 175 mg/dL or 60 mmol/L), chronic kidney disease (CKD as compared with acute kidney injury (AKI)), severe metabolic acidosis, older age, pediatric patients, pre-existing neurologic disease (head trauma, stroke, seizure disorder), conditions characterized by cerebral edema (hyponatremia, hepatic encephalopathy, malignant hypertension), and any condition that increases the permeability of the blood brain barrier (sepsis, vasculitis, thrombotic thrombocytopenic purpura-hemolytic uremic syndrome, encephalitis, or meningitis) [2-6]. DDS can present with a wide spectrum of clinical manifestations, both mild and severe. Mild symptoms of DDS include nausea, vomiting [1], headache, anorexia, blurred vision, muscle cramps, disorientation, restlessness, hypertension and dizziness. More severe symptoms of DDS are consistent with central nervous system dysfunction, such as seizures, central pontine myelinolysis, and ultimately coma and death. DDS has been credited for acute electroencephalographic abnormalities and structural changes on diagnostic imaging following rapid hemodialysis.
There are three theories proposed to explain the pathogenesis of DDS: the reverse urea effect, idiogenic osmoles hypothesis, and paradoxical brain acidosis. Kennedy et al first suggested the reverse urea effect after it was noted in a mouse model that urea was removed more slowly from cerebral spinal fluid than from blood during intermittent renal replacement therapy (IRRT) [7]. IRRT leads to rapid reduction in plasma urea with delay in the brain urea clearance creating an osmotic gradient between the plasma and brain [8, 9]. The discovery of urea transporters further supported the reverse urea effect because these transporters are down regulated in uremic states, thereby decreasing the ability of brain to quickly adapt to the plasma urea loss.
The idiopathic osmoles hypothesis suggests that dialysis may generate other, but thus far unidentified, osmotically active molecules that contribute to brain edema [10].
Finally, paradoxical brain acidosis suggests that as renal replacement therapy (RRT) corrected the acidosis, brain pH decreased, thereby creating an osmotic gradient by displacement of intracellularly protein bound potassium and sodium. Consequently, these ions become osmotically active causing influx of water [11].
In our case patient had risk factors like severe sepsis, septic shock, increased BUN, and severe metabolic acidosis, which have altered the blood brain barrier and may have contributed for the development of DDS.
As DDS is the development of an osmotic gradient causing water to move into the brain, preventing the development of this gradient should prevent the syndrome. The simplest way to do this is to perform hemofiltration on the patient instead of dialysis. This method of treatment relies on the convective removal of solute from the patient in place of diffusive removal. Thus, the osmolalities of the body fluid compartments will not change as rapidly as they do during standard hemodialysis. This method was shown to reduce some of the symptoms that are related to the disequilibrium syndrome.
Conclusion
If a patient with severe sepsis and septic shock and with acute kidney failure who needs dialysis may develop DDS even after repeated sessions of the hemodialysis. DDS might have contributed to sudden deterioration and death in this young patient with septic shock.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this manuscript. This is to state that there has been no activity or involvements that will raise the question of bias in this case report or any of the conclusions or opinions that it stands for.
| References | ▴Top |
- Shaikh N, Louon A, Hanssens Y. Fatal dialysis disequilibrium syndrome: A tale of two patients. J Emerg Trauma Shock. 2010;3(3):300.
doi pubmed - Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int. 1994;45(3):629-635.
doi pubmed - Zepeda-Orozco D, Quigley R. Dialysis disequilibrium syndrome. Pediatr Nephrol. 2012;27(12):2205-2211.
doi pubmed - Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial. 2008;21(5):493-498.
doi pubmed - Bagshaw SM, Peets AD, Hameed M, Boiteau PJ, Laupland KB, Doig CJ. Dialysis Disequilibrium Syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure--a case report. BMC Nephrol. 2004;5:9.
doi pubmed - Marshall MR, Golper TA. Low-efficiency acute renal replacement therapy: role in acute kidney injury. Semin Dial. 2011;24(2):142-148.
doi pubmed - Kennedy A, Linton A, Eaton J. Urea levels in cerebrospinal fluid after hemodialysis. The Lancet. 1962;279(7226):410-411.
doi - Silver SM, DeSimone JA, Jr., Smith DA, Sterns RH. Dialysis disequilibrium syndrome (DDS) in the rat: role of the "reverse urea effect". Kidney Int. 1992;42(1):161-166.
doi pubmed - Rosen SM, O'Connor K, Shaldon S. Haemodialysis Disequilibrium. Br Med J. 1964;2(5410):672-675.
doi pubmed - Arieff AI, Massry SG, Barrientos A, Kleeman CR. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int. 1973;4(3):177-187.
doi pubmed - Arieff AI, Guisado R, Massry SG, Lazarowitz VC. Central nervous system pH in uremia and the effects of hemodialysis. J Clin Invest. 1976;58(2):306-311.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
