| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Review
Volume 9, Number 2, December 2020, pages 33-34
Effect of Magnesium Administration in Dextrose Water on Serum Sodium Concentration
Ramin Sam
Division of Nephrology, Zuckerberg San Francisco General Hospital and University of California, San Francisco, CA 94110, USA
Manuscript submitted May 28, 2020, accepted August 3, 2020, published online November 25, 2020
Short title: Magnesium Administration and Serum Na
doi: https://doi.org/10.14740/wjnu409
- Abstract
- Introduction
- Why Does Potassium Administration Not Lower Serum Sodium Concentration?
- What to Expect With Magnesium Administration?
- Conclusions
- References
| Abstract | ▴Top |
There is fair amount of data that potassium administration will raise serum sodium concentration; however whether the above concept extends to other electrolytes is less well understood. Although real-world data are lacking on this concept, here we will discuss the reasons why one may logically conclude that magnesium administration in dextrose-containing solution does not in fact lower serum sodium concentration appreciably. Furthermore we will try to determine by how much one should expect the administration of magnesium intravenously will change the serum sodium concentration.
Keywords: Magnesium administration; Dextrose-containing solution; Serum sodium concentration; Intravenously
| Introduction | ▴Top |
Recently a 51-year-old man with history of diabetes and human immunodeficiency virus (HIV) infection was sent to the emergency room after finding abnormal labs during a routine primary care visit. Labs indicated a serum sodium concentration of 118 mEq/L, potassium 2.9 mEq/L, calcium 8.2 mg/dL, magnesium 1.5 mg/dL, and phosphorus of 1.7 mg/dL. Subsequently serum magnesium concentration dropped to as low as 1.1 mg/dL and there was concern that giving several magnesium drips in dextrose 5% in water (D5W) will lead to worsening of the hyponatremia because of the hyponatremic solution. Magnesium infusions come in 100 mL bag of D5W and contain 1 g of magnesium sulfate.
When one supplements potassium in a patient with hyponatremia and hypokalemia, the prevailing consensus is that the potassium infusion even if in D5W will not lower the serum sodium concentration as there is a preponderance of data that potassium administration will not decrease serum sodium concentration [1, 2]. The effect of magnesium administration on serum sodium concentration is however much less clear. Here an argument will be made that magnesium administration will not lower serum sodium concentration significantly even if contained in D5W.
| Why Does Potassium Administration Not Lower Serum Sodium Concentration? | ▴Top |
When one administers potassium chloride, the potassium will mainly go intracellularly as it is the main intracellular cation. The movement of the potassium intracellularly will drag water from the plasma/interstitial space into the cell along with potassium in order to maintain osmolality the same in all three compartments. As sodium does not enter the cell along with the water, the plasma sodium concentration will rise as the plasma is concentrated [3, 4].
As seen in Figure 1, if one assumes total body water of 40 L with two-third being intracellular and one-third extracellular, then if one administers 40 mEq of potassium chloride in 100 mL of D5W (assuming that all of the potassium chloride goes intracellularly), then the serum sodium concentration would increase from 142 mEq/L to 142.55 mEq/L.
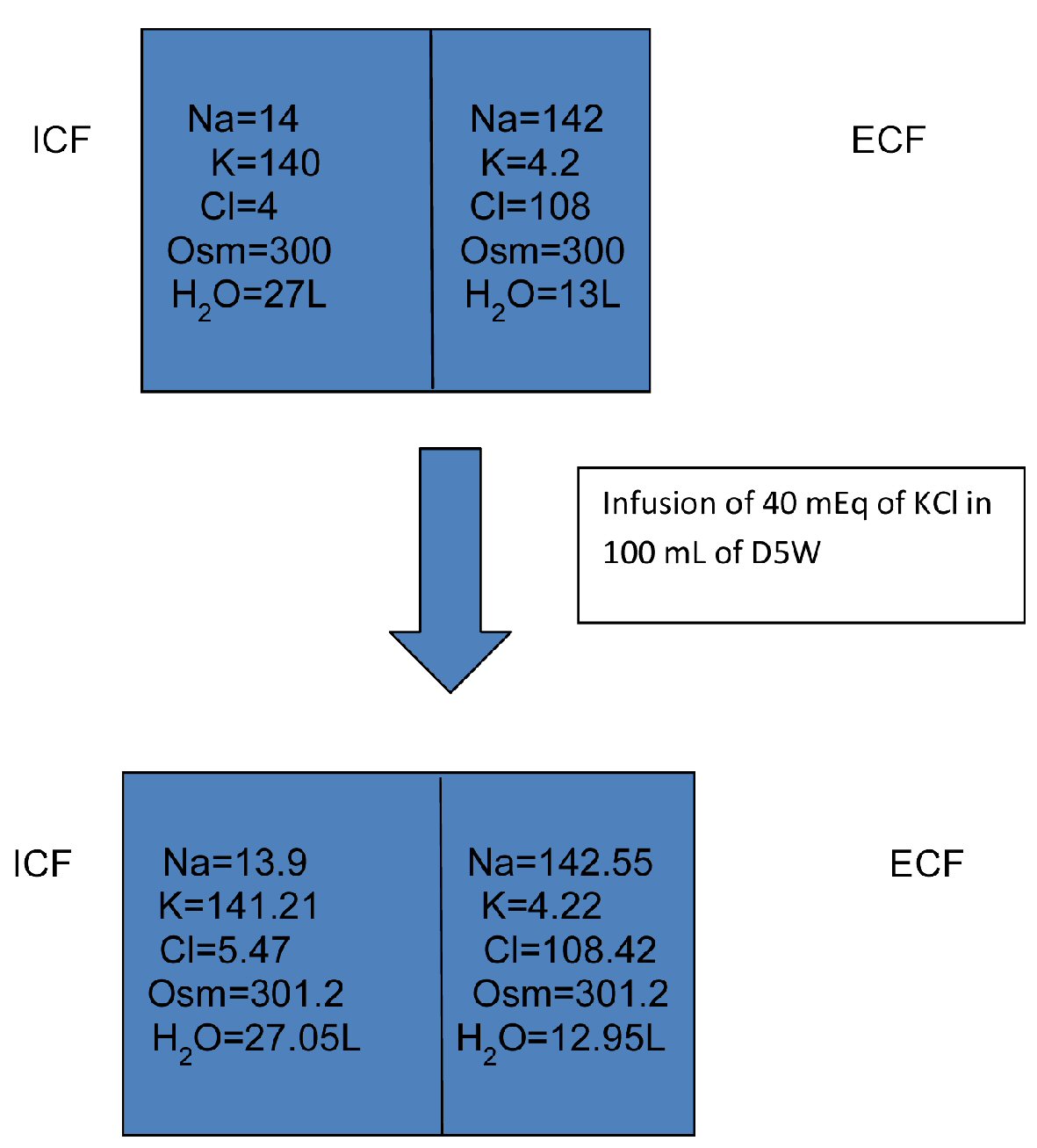 Click for large image | Figure 1. Infusion of 40 mEq of KCl in 100 mL of D5W. D5W: dextrose 5% in water; ICF: intracellular fluid; ECF: extracellular fluid; KCl: potassium chloride. |
| What to Expect With Magnesium Administration? | ▴Top |
Magnesium is mainly intracellular also (second most abundant intracellular cation) with less than 1% of the ion being present in the extracellular fluid [5, 6]. It is also highly concentrated in the skeletal muscle cells. Again if magnesium administration will lead to nearly all of the administered magnesium to move intracellularly, then water will follow and there would be an increase in serum sodium concentration countered by the dilution of sodium caused by dextrose in water infusion.
Figure 2 depicts how administration of 2 g of magnesium sulfate in 200 mL of D5W will change the intracellular and extracellular concentration of different ions (the magnesium concentrations are in mmol/L). Here with administration of 2 g of magnesium sulfate in 200 mL of D5W, the serum sodium concentration barely changed (142 mEq/L to 141.7 mEq/L).
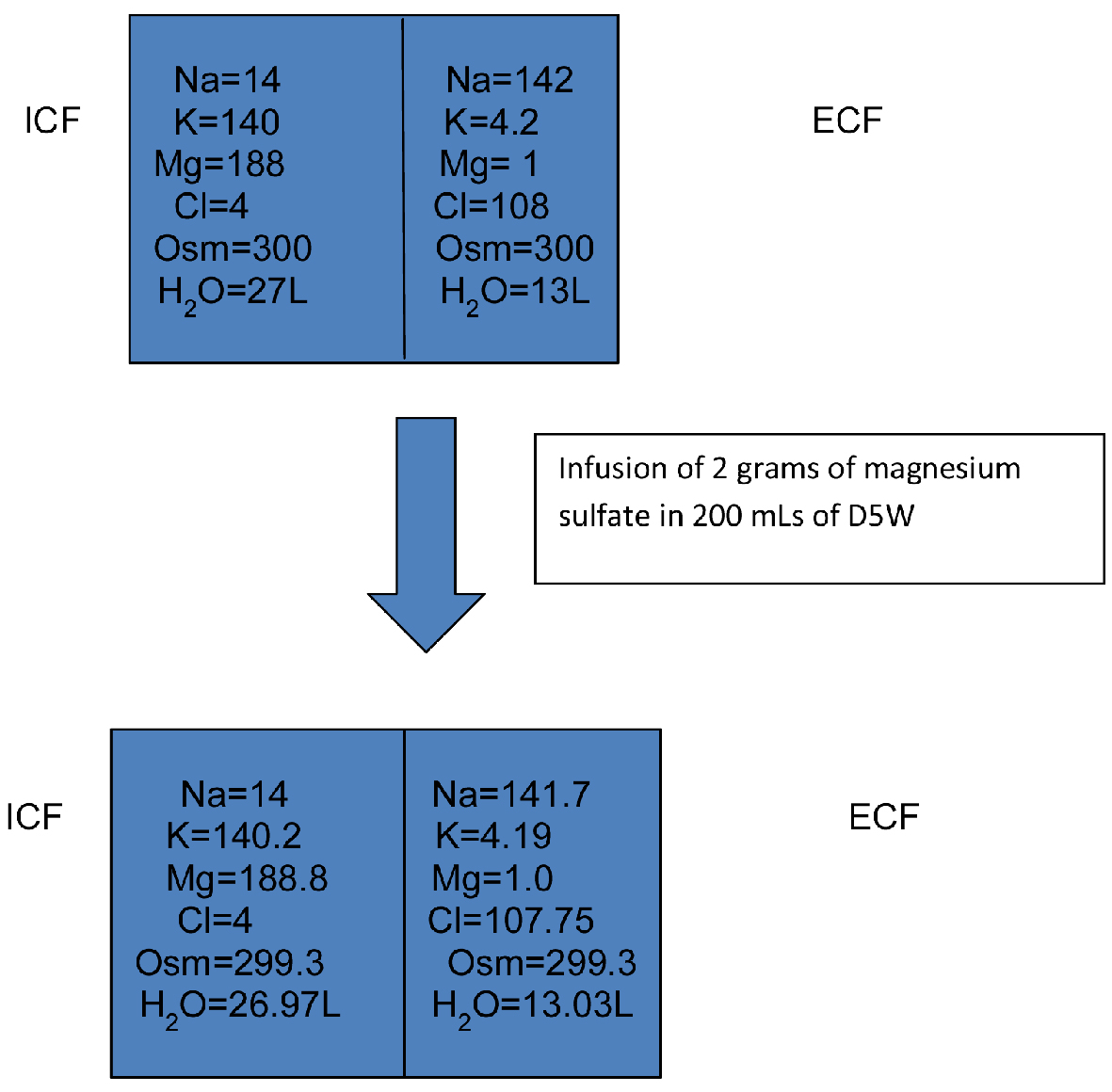 Click for large image | Figure 2. Infusion of 2 g of magnesium sulfate in 200 mL of D5W. D5W: dextrose 5% in water; ICF: intracellular fluid; ECF: extracellular fluid. |
| Conclusions | ▴Top |
Administration of magnesium sulfate intravenously contained in dextrose in water will not result in significant lowering of the serum sodium concentration, as the infusion of dilute fluid is counterbalanced by concentration of the extracellular fluid from movement of the magnesium intracellularly and dragging of water along with it. Physician can correct the serum magnesium levels safely with magnesium riders without worry that the serum sodium concentration will fall extensively.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Edelman IS, Leibman J, O'Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236-1256.
doi pubmed - Laragh JH. The effect of potassium chloride on hyponatremia. J Clin Invest. 1954;33(5):807-818.
doi pubmed - Sam R, Feizi I. Understanding hypernatremia. Am J Nephrol. 2012;36(1):97-104.
doi pubmed - Sam R. Osmolality and serum sodium concentration. In: Sam R, Lo L, Gluck SL. Understanding basic renal pathophysiology. New York: Nova Science Publishers, Inc. 2018. p. 31-55.
- Murphy E. Mysteries of magnesium homeostasis. Circ Res. 2000;86(3):245-248.
doi pubmed - Zaloga GP. Interpretation of the serum magnesium level. Chest. 1989;95(2):257-258.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
