| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Original Article
Volume 11, Number 1, June 2022, pages 1-9
Anticancer Effect of Medicinal Mushroom Extract on Renal Cell Carcinoma: Alternative Therapeutic Implication
Ashley Dixona, Jason Elyaguova, Muhammad Choudhurya, Sensuke Konnoa, b
aDepartment of Urology, New York Medical College, Valhalla, New York 10595, USA
bCorresponding Author: Sensuke Konno, Department of Urology, New York Medical College, Valhalla, NY 10595, USA
Manuscript submitted May 11, 2022, accepted May 27, 2022, published online June 2, 2022
Short title: Anticancer Effect of Poria Mushroom Extract
doi: https://doi.org/10.14740/wjnu433
| Abstract | ▴Top |
Background: To investigate if medicinal Poria mushroom extract (PE) would be a potential therapeutic agent against renal cell carcinoma (RCC) as it has been shown to have anticancer/antitumor activity with few side effects. We examined its anticancer effect on RCC as well as its potential anticancer mechanism in vitro.
Methods: Anticancer effect of PE was first assessed by the dose-dependent effects (0 - 200 µg/mL) on human RCC, ACHN cells. Its anticancer mechanism was then explored focusing on specific biochemical and molecular parameters
Results: PE concentrations ≥ 100 µg/mL led to 50-90% reduction in cell viability, accompanied by a significant decrease in hexokinase activity and cellular adenosine triphosphate (ATP) level, implying the inhibition of glycolysis. The key metabolic regulators, adenosine monophosphate (AMP)-activated protein kinase (AMPK), protein kinase B (Akt), and mammalian target of rapamycin (mTOR), were specifically modulated by PE. These results were indicative of subsequent growth cessation and cell death. Cell cycle analysis also revealed a G1 cell cycle arrest and the upregulation of activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), and glucose-regulated protein 78 (GRP78), indicated induction of endoplasmic reticulum (ER) stress. Ultimately, such ER stress led to apoptosis, evidenced by proteolytic activation of caspase-3 (Csp-3) and poly-ADP ribose polymerase (PARP).
Conclusions: PE has anticancer effect on ACHN cells, resulting in the significant reduction in cell viability. Its anticancer mechanism appears to be linked to glycolysis inhibition, modulations of metabolic pathways, and ER stress-induced apoptosis.
Keywords: Renal cell carcinoma; ACHN cells; Medicinal mushroom; Anticancer; Apoptosis
| Introduction | ▴Top |
Renal cell carcinoma (RCC) is a heterogenous cluster of cancers arising from the nephron in the kidney [1] and is the third most common genitourinary tumor and the eighth common neoplasm, which has steadily increased in the past 30 years. In 2022, approximately 79,000 new cases and nearly 14,000 deaths were estimated in the USA [2], and almost 25-30% of those patients with RCC have presented with metastatic disease at the time of diagnosis [3, 4]. Clear cell RCC is the most prevalent and making up about 70% of RCC [1], and standard treatment for non-metastatic RCC is complete resection of the tumor by either a radical or partial nephrectomy [5]. Currently, less invasive approaches, such as laparoscopic and robotic-assisted nephrectomies, are routinely used in partial nephrectomy [6]. However, 20-30% of patients after surgery [7, 8] would progress to a metastatic disease with the 5-year survival rate of merely 8-10% [1, 8], which is devastating and dismal. Particularly, patients with positive margins after surgery are more likely to relapse locally or develop distant metastasis [9]. Although other viable options, such as immunotherapy, radiotherapy, chemotherapy, etc. [3, 10], are currently available, no effective modalities have been established yet. Immunotherapy has some good outcomes but suffers from a high dose, high cost, and considerable side effects [11]. Incidentally, monotherapy of interferon-α2b (IFN-α2b) or interleukin-2 (IL-2) had only the response rates of 10-20% and showed a small improvement in survival [8]. To improve the efficacy, the combination of nephrectomy and immunotherapy (IFN-α2b or IL-2) was then attempted. Nephrectomy followed by IFN-α2b, or IL-2 infusion showed the improved outcomes with longer survival than immunotherapy alone [12], although whether the high doses of these cytokines would be yet tolerable and durable needs to be clarified. Nevertheless, more studies are urgently demanded because the improved modality bringing out satisfactory outcomes needs to be established and metastatic RCC yet remains substantially resistant to systemic therapy.
Recently, altered or dysregulated metabolic pathways have been proposed to play a significant role in the development and progression of RCC [13]. Glycolysis is one of essential metabolic processes to generate cellular energy (adenosine triphosphate (ATP)) required for cellular function, proliferation, and survival [14, 15]. Peculiarly, cancer cells preferentially follow inefficient aerobic glycolysis (even in the presence of oxygen), instead of more efficient oxidative phosphorylation (via tricarboxylic acid (TCA) cycle), for ATP generation. This phenomenon is known as Warburg effect [16], which has not been fully understood. Nonetheless, it is suggested that oncogene activation or loss of tumor suppressor function may lead to such a metabolic shift or alteration towards aerobic glycolysis [16]. As targeting glycolysis could be a potential therapeutic strategy [16, 17], we have been exploring natural products/agents with anticancer activity capable of disrupting glycolysis.
We then came across Poria mushroom, which was not just a new edible mushroom but one of well-established medicinal mushrooms used for 2,000 years in traditional Chinese medicine [18]. Its bioactive extract, Poria mushroom extract (PE), was found to have the major chemical constituents including triterpenes, polysaccharides, and steroids [18]. Extensive scientific/medical studies have also identified its medicinal/pharmacological properties such as anticancer/antitumor, immunomodulatory, antioxidant, and renoprotective effects [19-21]. Additionally, PE is a natural agent with few side effects, having potential clinical implications in cancer treatment.
Accordingly, we investigated if PE might exhibit anticancer effect on RCC in vitro. To explore the anticancer mechanism, we particularly focused on several key cellular/biochemical events, such as glycolysis, metabolic signaling pathways, cell cycle, endoplasmic reticulum (ER) stress, and apoptosis. More details are described, and interesting findings are also discussed herein.
| Materials and Methods | ▴Top |
Cell culture
The human RCC ACHN cells (with aggressive grade IV property) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 µg/mL). Poria mushroom extract, PE, was a generous gift from the manufacturer (Mushroom Wisdom, Inc., East Rutherford, NJ). For experiments, cells were seeded at the initial cell density of 2 × 105 cells/mL in the six-well plates or T-75 flasks and cultured with varying concentrations of PE. Cell viability was then assessed at 72 h by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay described below.
Cell viability test (MTT assay)
Cells treated with PE for 72 h were subjected to MTT assay, following the vendor’s protocol (Sigma-Aldrich, St. Louis, MO). At the harvest time (at 72 h), 1 mL of MTT reagent (1 mg/mL) was added to each well in the six-well plate, followed by 3-h incubation at 37 °C. After removing MTT reagent, dimethyl sulfoxide was added to each well to dissolve formazan precipitates (purple color) and absorbance was read on a microplate reader. Cell viability was then expressed by the percent (%) of viable cells relative to the control reading (100%).
Cell cycle analysis
A BD FACscan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ), equipped with a double discrimination module, was employed for cell cycle analysis. Cells (about 1 × 106 cells) were resuspended in propidium iodide solution and incubated for 1 h at room temperature. Approximately 10,000 nuclei from each sample were analyzed on a flow cytometer, and CellFit software was used to quantify cell cycle compartments to estimate the % of cells distributed in the different cell cycle phases.
Hexokinase (HK) assay
HK activity was determined using the HK colorimetric assay kit (BioVision, Waltham, MA) following the manufacturer’s protocol. Cell lysates (20 µg per sample) and NADH standards were prepared in the 96-well plate and the reaction was started by the addition of reaction mixture (containing substrate). The plate was placed in a microplate reader and the absorbance (OD) changes with time were monitored at 450 nm for 20 min with 5-min intervals. All readings were calculated using references (NADH standards) and HK activity was expressed by the % of sample activity relative to the controls (100%).
Determination of cellular ATP level
The cellular ATP level was determined using the ATP colorimetric assay kit (BioVision) following the vender’s protocol. Cells (2 × 105 cells/mL) in the six-well plate were first lysed in ATP assay buffer, deproteinized with HClO4, and neutralized with KOH. Cell lysates (50 µL per sample) and ATP standards were prepared in the 96-well plate and the reaction was started by the addition of reaction mixture. The plate was then incubated at room temperature for 30 min in the dark. Absorbance at 570 nm was read on a microplate reader and ATP contents in samples were calculated by referring the sample readings to the readings of ATP standards. The ATP level was then expressed by the % of ATP amounts in samples relative to the controls (100%).
Western blot analysis
Briefly, an equal amount of cell lysates (10 µg) was first subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blot (membrane) was incubated for 90 min with primary antibodies against three metabolic regulators, adenosine monophosphate (AMP)-activated protein kinase (AMPK), protein kinase B (Akt), and mammalian target of rapamycin (mTOR), three stress-related factors, activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), and glucose-regulated protein 78 (GRP78), and also two apoptotic regulators, caspace-3 (Csp-3) and poly-ADP ribose polymerase (PARP) (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with secondary antibody conjugates for 30 min. Specific immunoreactive protein bands were then detected by chemiluminescence following vender’s protocol (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Statistical analysis
All data are presented as mean ± standard deviation (SD), and statistical differences between groups are assessed with one-way analysis of variance (ANOVA). Values of P < 0.05 are considered to indicate statistical significance
The Institutional Review Board (IRB) approval and ethical compliance was not required as no humans or animals involved in this study.
| Results | ▴Top |
Anticancer effect of PE on ACHN cells
Anticancer effect of PE could be assessed by cell viability, indicating how many cells (%) are yet alive/viable relative to control cells (100%) following PE treatment. ACHN cells were cultured with varying concentrations of PE (0 - 200 µg/mL) and cell viability was determined in 72 h by MTT assay. Although PE at 50 µg/mL had little effect, PE concentrations ≥ 100 µg/mL showed a significant (P < 0.05) reduction in cell viability, such as about 50% reduction with 100 µg/mL (Fig. 1). Thus, these results suggest that PE appears to have anticancer effect capable of significantly reducing cell viability in ACHN cells. Since PE (100 µg/mL) is proximate to its 50% inhibitory concentration (IC50) or shows its 50% anticancer effect, this concentration was then used in the rest of our study.
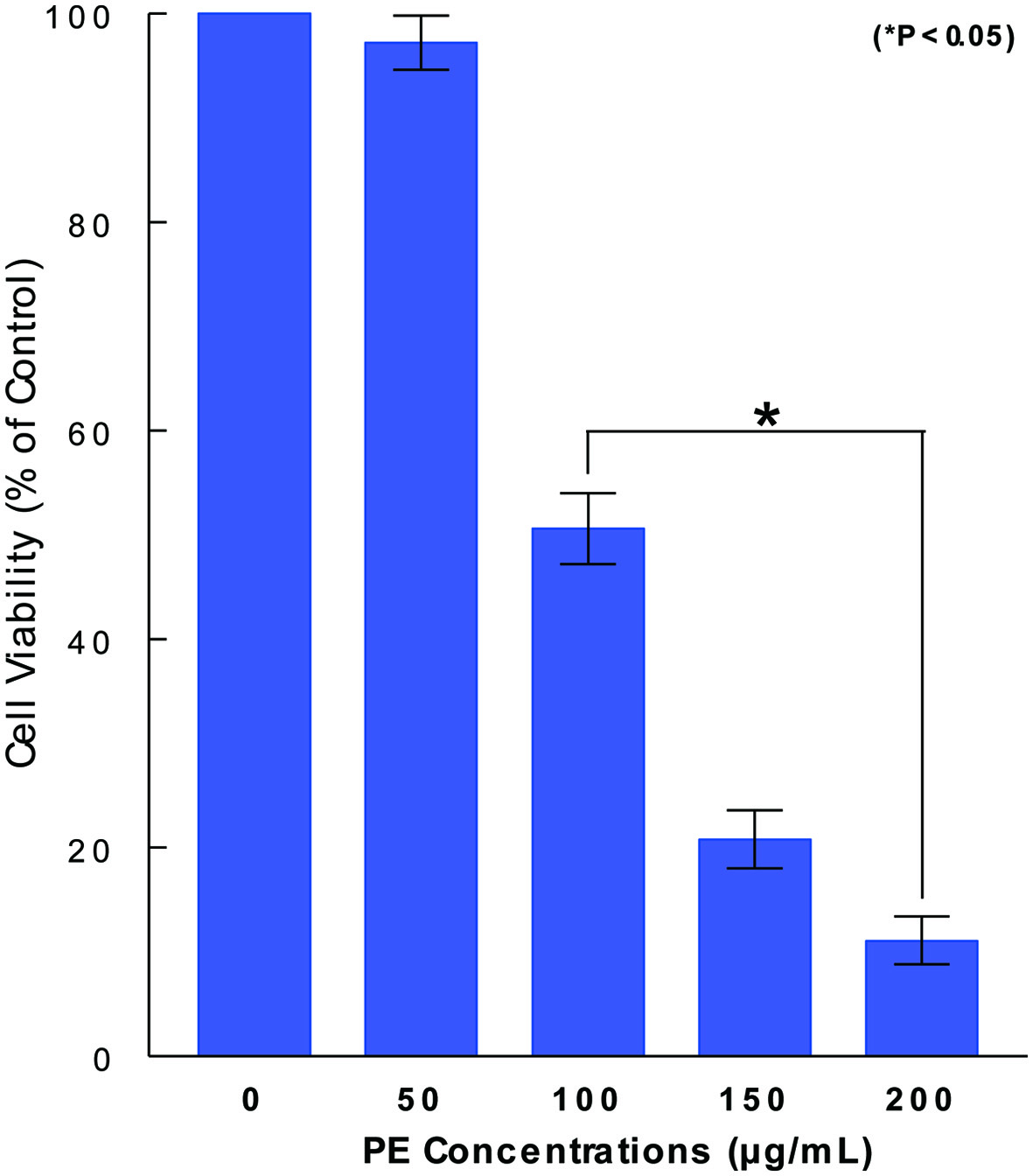 Click for large image | Figure 1. Dose-dependent effect of PE on ACHN cell viability. ACHN cells were cultured with varying concentrations of PE (0 - 200 µg/mL) and cell viability was assessed in 72 h by MTT assay. Cell viability was expressed by the percent (%) of viable cells relative to controls (100%). The data are mean ± standard deviation (SD) from three separate experiments (*P < 0.05 compared with control). PE: Poria mushroom extract; MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide. |
Inhibitory effect of PE on glycolysis
To have an insight into how PE induces such a significant cell viability reduction, we examined if glycolysis might be affected/inhibited by PE because it is the vital metabolic process required for cell proliferation and survival [15]. We specifically assessed the status of one of key glycolytic enzymes, HK, that is involved in the irreversible committed step in glycolysis [22]. Cells were treated with PE (100 µg/mL) for 72 h and subjected to HK assay. Compared to controls (100%), HK activity declined to about 60% (i.e., about 40% activity loss) with PE treatment (Fig. 2). Since this HK inhibition could shut down the rest of glycolytic pathway, the yield of the end product (i.e., ATP) would be also reduced. This possibility was then tested. Assay showed that the ATP level was indeed reduced to about 45% (i.e., about 55% reduction) with PE, further confirming the inhibition or incompletion of glycolysis (Fig. 2). Thus, these results suggest that the cell viability reduction induced by PE is at least in part attributed to the glycolysis inhibition, evidenced by the reduction in HK activity and ATP synthesis with PE.
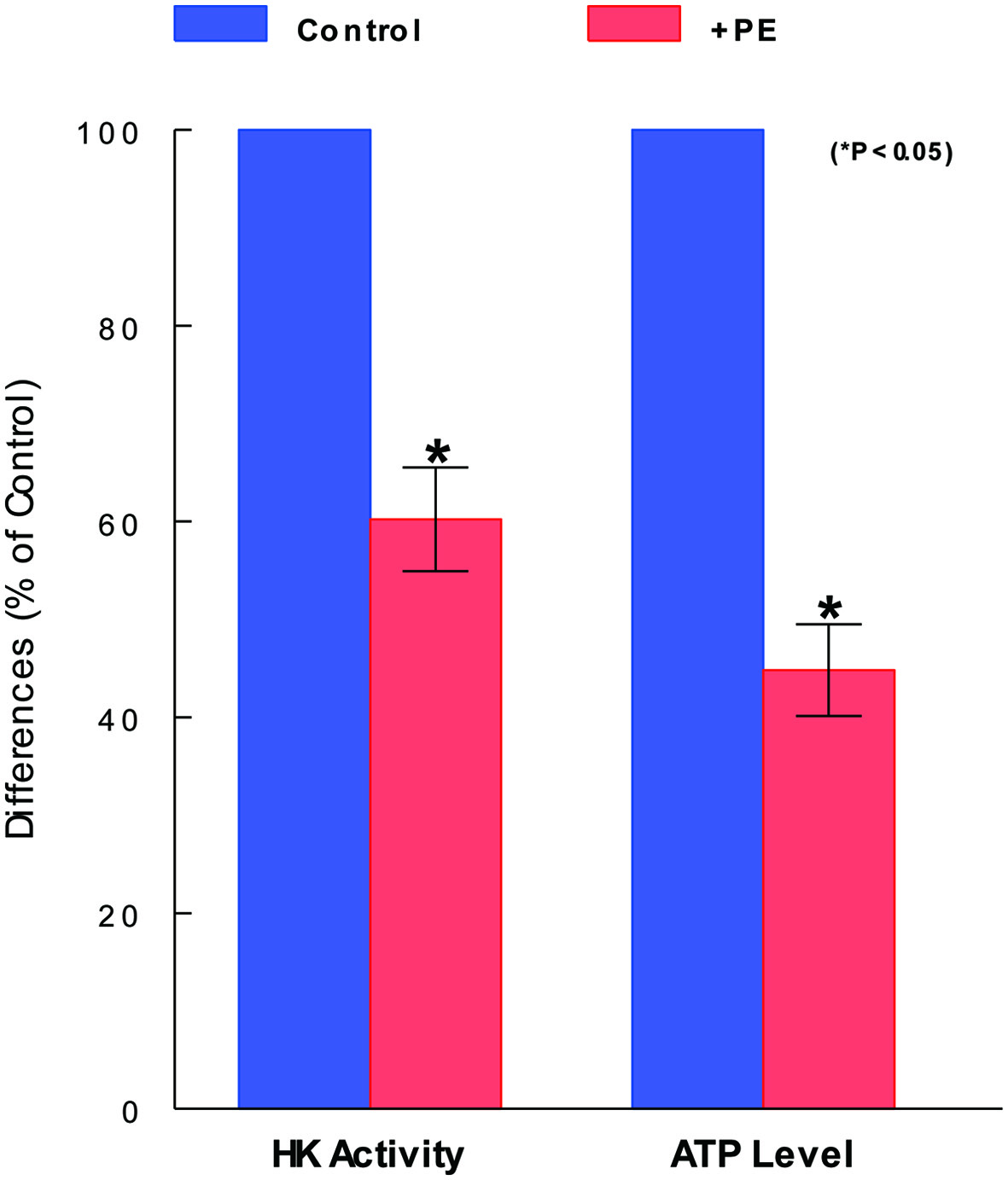 Click for large image | Figure 2. Inhibitory effects of PE on glycolytic parameters. After cells were treated with PE (100 µg/mL) for 72 h and subjected to HK and ATP assays separately. HK activity and ATP level are expressed by the % relative to respective controls (100%). The data are mean ± standard deviation (SD) from three separate experiments (*P < 0.05 compared with control). PE: Poria mushroom extract; HK: hexokinase; ATP: adenosine triphosphate. |
Effects of PE on metabolic signaling pathways
As such a glycolysis inhibition would be linked to the metabolic signaling pathways [23], cells treated with PE for 72 h were analyzed for three key metabolic regulators using Western blots. Such analysis revealed that AMPK [23, 24] was highly phosphorylated (activated), while Akt [25] and mTOR, working together to promote cell proliferation [26], were dephosphorylated (inactivated) with PE (Fig. 3). Thus, such a profile of activation of AMPK concomitant with inactivation of Akt/mTOR by PE rather suggests that PE could ultimately induce cell death in ACHN cells, as reported in other cancer cells elsewhere [23-25].
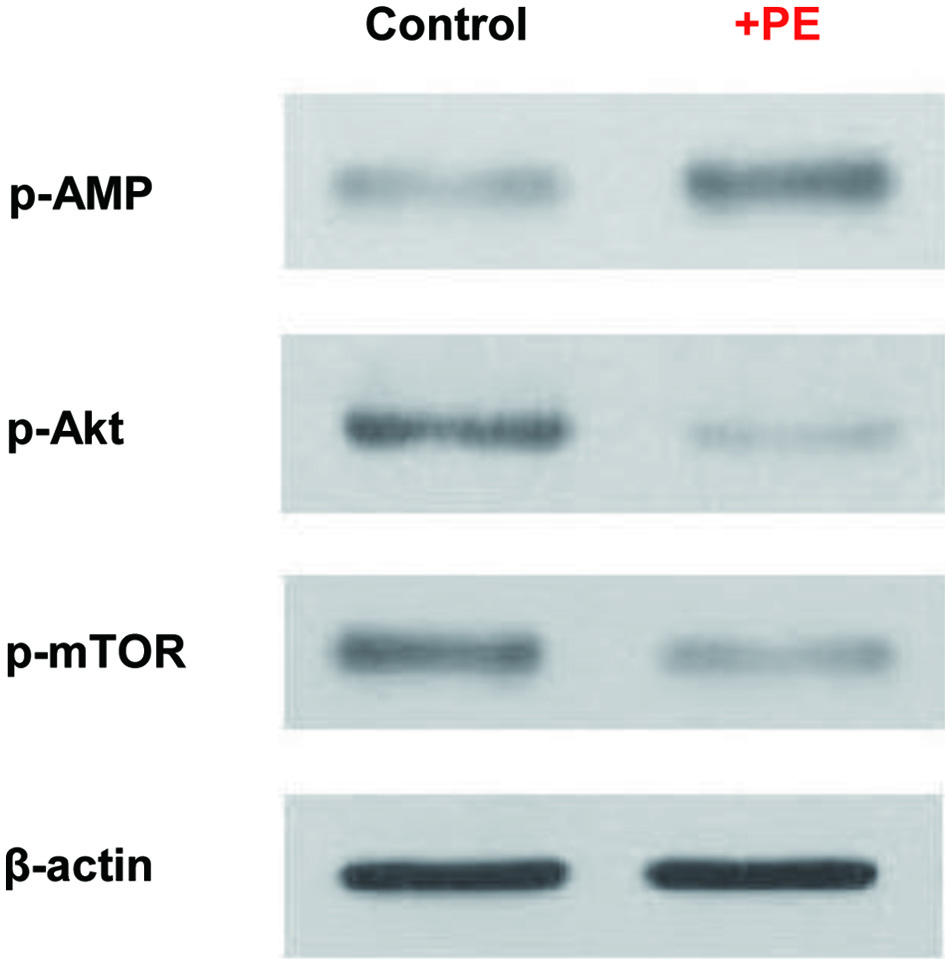 Click for large image | Figure 3. Modulations of metabolic regulators by PE. Cells treated with PE (100 µg/mL) for 72 h were analyzed for four key metabolic regulators using Western blots. Autoradiographs of p-AMPK, p-Akt, and p-mTOR expressed in control and PE-treated cells are shown for comparison. Beta-actin is also shown as a loading control. PE: Poria mushroom extract; AMPK: AMP-activated protein kinase; Akt: protein kinase B; mTOR: mammalian target of rapamycin. |
Effect of PE on cell cycle
It has been also shown that energy (ATP) reduction/depletion (due to the glycolysis inhibition) would subsequently affect cell cycle, perhaps resulting in a cell cycle arrest [27]. Accordingly, cells treated with PE for 72 h were subjected to cell cycle analysis, and the results showed that PE led to about 37% increase in G1-phase cell number/population concomitant with about 44% decrease in S-phase cell number, compared to those in controls (Fig. 4). This cell accumulation in the G1 phase is known as a G1 cell cycle arrest [28], due to a blocking of cells entering from the G1 to the S phase. Thus, PE appears to interrupt the cell cycle progression, eventually leading to the growth cessation and cell viability reduction.
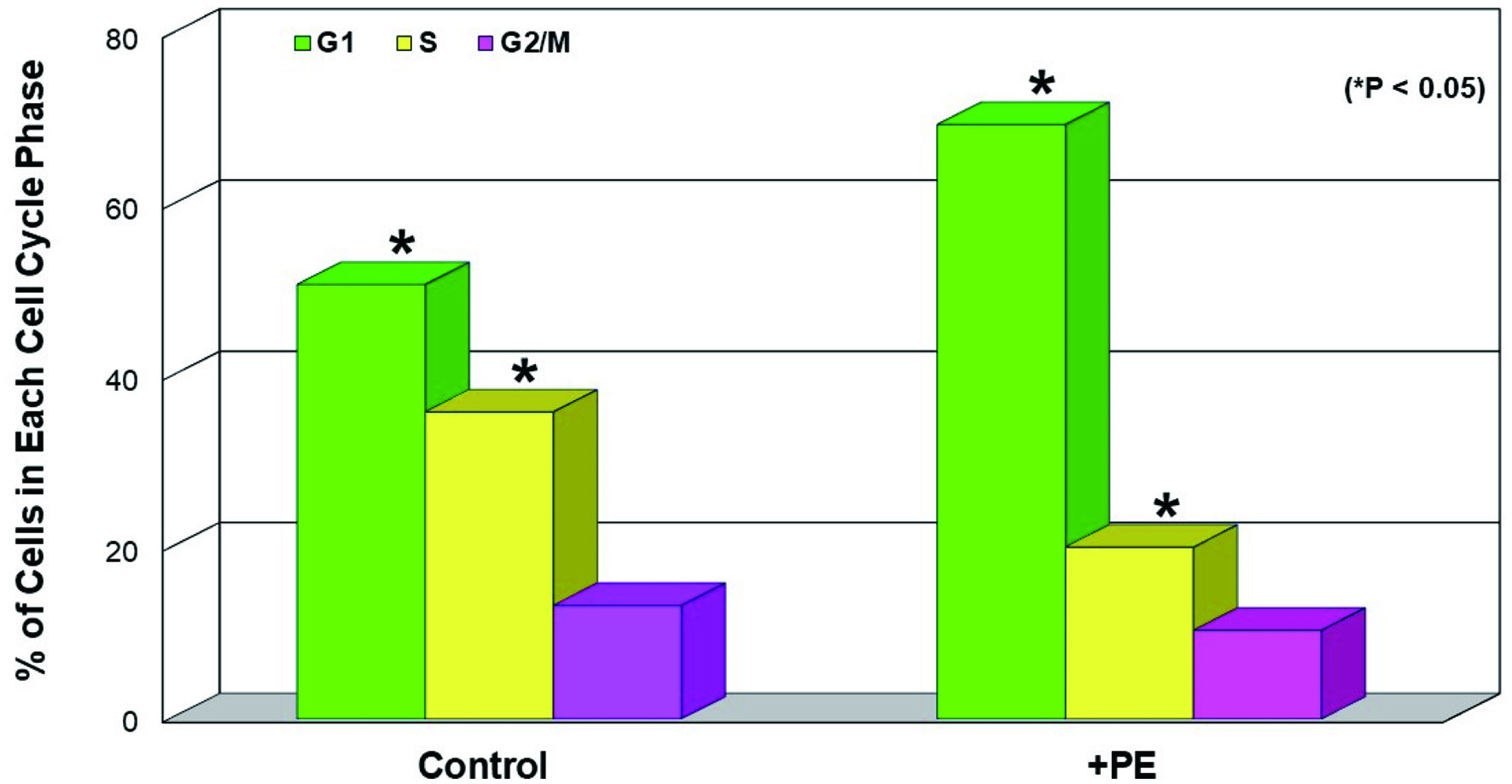 Click for large image | Figure 4. Induction of G1 cell cycle arrest by PE. Cells treated with PE (100 µg/mL) for 72 h were subjected to cell cycle analysis and the % of cell distribution at each cell cycle phase in control and PE-treated cells was plotted. The data are mean ± SD from three separate experiments but only mean values are shown (*P < 0.05 compared with control). PE: Poria mushroom extract; SD: standard deviation. |
Induction of ER stress by PE
In addition, a G1 cell cycle arrest has been reported to be also induced by ER stress [29], in which the increased amount of unfolded and misfolded proteins is accumulated in ER, disrupting ER homeostasis [30]. Cells treated with PE for 72 h were subjected to Western blot analysis to assess the protein expressions of two ER stress-related transcriptional factors, ATF4 and CHOP [31, 32], as well as an ER regulator, GRP78 [33]. The results then revealed that compared to controls, ATF4, CHOP, and GRP78 were all upregulated or highly expressed in PE-treated cells (Fig. 5), indicating cells under ER stress. Thus, it is plausible that PE-induced ER stress is presumably linked to a G1 cell cycle arrest observed earlier (Fig. 4).
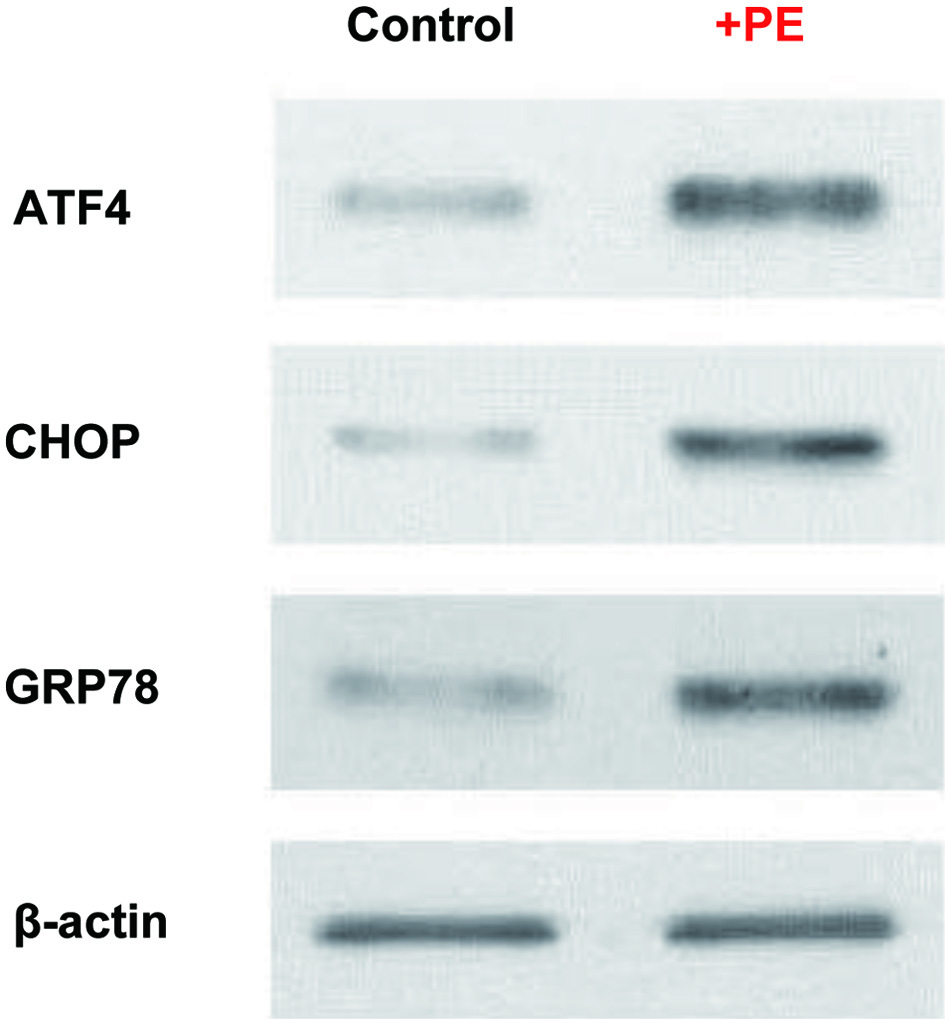 Click for large image | Figure 5. PE induced ER stress. Cells treated with PE (100 µg/mL) for 72 h were analyzed for three ER-related factors, ATF4, CHOP, and GRP78, using Western blots. Autoradiographs of these three factors expressed in control and PE-treated cells are shown for comparison. Beta-actin is also shown as a loading control. PE: Poria mushroom extract; ATF4: activating transcription factor 4; CHOP: C/EBP homologous protein; GRP78: glucose-regulated protein 78. |
ER stress-induced apoptosis by PE
Since ER stress has been shown to ultimately induce apoptosis [34], this possibility was then examined. After cells were treated with PE for 72 h, the status of two apoptotic regulators, caspase-3 (Csp-3) [35] and PARP [36], were assessed by Western blots. Analysis revealed that PE treatment led to the upregulation or activation of both Csp-3 and PARP, indicated by appearance of the cleaved/fragmented protein bands through proteolysis (Fig. 6). Such a cleavage of protein band is indicative of proteolytic activation of Csp-3 and PARP, which has been shown to eventually induce apoptosis [35, 36]. Therefore, these results demonstrate that PE-induced ER stress would ultimately induce apoptosis in ACHN cells.
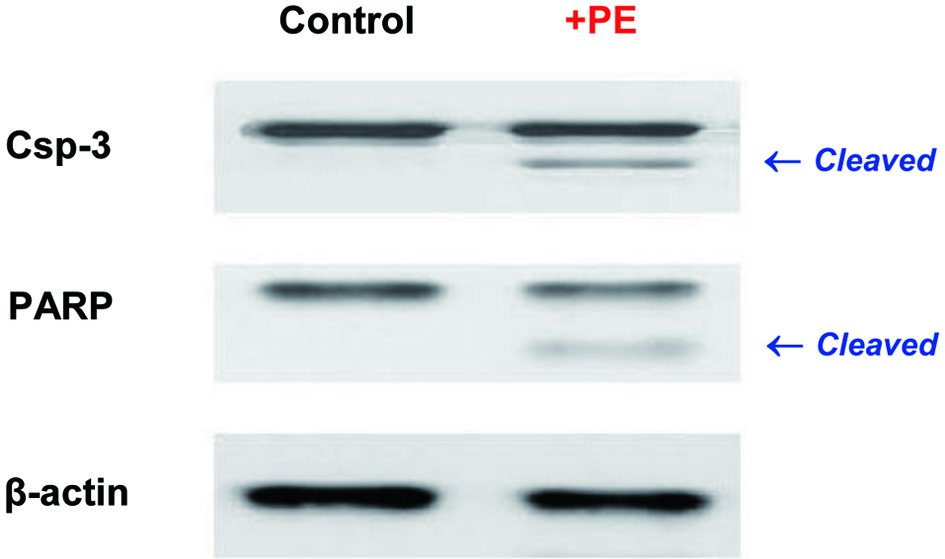 Click for large image | Figure 6. Induction of apoptosis. Cells treated with PE (100 µg/mL) for 72 h were analyzed for two apoptotic regulators using Western blots. Autoradiographs of Csp-3 and PARP in control and PE-treated cells are shown for comparison. The small, cleaved bands by proteolysis in PE-treated cells are indicative of active forms. Beta-actin is also shown as an internal control. PE: Poria mushroom extract; Csp-3: caspace-3; PARP: poly-ADP ribose polymerase. |
| Discussion | ▴Top |
To improve prognosis and survival rate of RCC, especially in patients with multiple tumors who undergo minimally invasive nephron sparing procedures and find an oral agent for more effective treatment, we investigated the potential anticancer effect of Poria mushroom extract, PE, on human renal carcinoma ACHN cells in vitro. We found that PE indeed had a potent anticancer effect with the IC50 of about 100 µg/mL. Similarly, it has been also reported that proliferation of human breast cancer MCF-7 cells was significantly reduced with PE, whose IC50 was estimated to be 400 µg/mL [37], demonstrating its anticancer effect against breast cancer cells. As mentioned earlier, PE has the bioactive chemical compounds such as triterpenes, polysaccharides, and steroids [18], which may play an essential role in anticancer activity. In fact, β-glucan, one of polysaccharides, was actually found to be responsible for anticancer effect against MCF-7 cells [37]. The similar β-glucan mediated anticancer effect was also demonstrated in other cancer cells. Proliferation of human leukemic U937 and HL-60 cells was inhibited with PE (β-glucan) and they were also differentiated to (normal) mature monocytes/macrophages expressing several characteristic features [38]. This is indeed the interesting finding showing antiproliferation and differentiation of leukemic cells to normal cells induced by PE.
To explore the anticancer mechanism, we examined the effect of PE on glycolysis, which was an essential metabolic process required for cell growth and survival [15]. The results showed that PE directly targeted HK, the key glycolytic enzyme to trigger and carry out the glycolytic pathway [22]. As a result, the rest of the pathway was shut down as evidenced by the fact that cellular ATP synthesis was also significantly diminished (Fig. 2). This is indicative of the inhibition of glycolysis by PE.
In conjunction with the reduced cellular ATP level, due to the glycolysis inhibition, the metabolic signaling pathways were also disrupted with PE, indicated by the modulations of three signaling regulators, AMPK, Akt, and mTOR [24-26] (Fig. 3). These findings would then more likely lead to the inhibition of cell proliferation or even cell death.
Additionally, a metabolic impediment with PE further resulted in a G1 cell cycle arrest [28] shown by a significant increase in G1-phase cell number concomitant with a significant decrease in S-phase cell number (Fig. 4). This cell cycle arrest would subsequently cease cell proliferation, possibly accounting for the PE-induced cell viability reduction. Similarly, one of specific triterpene derivatives of PE (dehydroebriconic acid) has been shown to induce a G1 cell cycle arrest via the inhibition of DNA topoisomerase II (topo II) in gastric cancer NUGC-3 cells [39]. Eventually, over 90% of cell growth was inhibited and the calculated IC50 was 38.4 µM. Hence, this specific PE derivative seems to be a novel topo II inhibitor and has potent anticancer activity.
Moreover, we found that the three key ER-related regulators, ATF4, CHOP, and GRP78, were all significantly upregulated by PE (Fig. 5), implying a disruption of ER homeostasis or ER stress [30]. In general, cells rapidly respond to such ER stress to correct or restore homeostasis through the unfolded protein response (UPR) by activating several transcription factors [30]. In particular, GRP78 plays a critical role in ER stress. It is known as a major chaperone in ER and a central sensor of cellular stress and highly expressed in most solid tumors [33], including the metastatic type of prostate cancer [40]. Such overexpression of GRP78 has been also shown to be associated with apoptosis resistance, immune escape, angiogenesis, and drug resistance [41]. However, if ER stress chronically persists and the accumulation of unfolded or misfolded proteins also continues, this overexpressed/activated GRP78 would then trigger and ensure the ER stress-induced apoptotic pathway [42].
In fact, we confirmed that cells indeed followed the apoptotic pathway under ER stress, evidenced by activation of two apoptotic regulators, Csp-3 [35] and PARP [36] (Fig. 6). This is a typical case of ER stress-induced apoptosis as proteolytic activation of these regulators has been shown to result in apoptosis [34]. Another triterpene derivative of PE, dehydrotrametenolic acid, has been also reported to induce apoptosis through activation of Csp-3 and PARP in H-ras-transformed rat2 cells and H-ras-expressing human bladder cancer J82 cells. A significant growth inhibition was seen in transformed rat2 cells and J82 cells with the IC50 of 39.2 and 26.7 µM, respectively [43]. However, neither growth inhibition nor apoptosis was observed in normal rat2 cells (missing H-ras). Thus, these results suggest that this PE derivative could be a potential anticancer agent against H-ras-transformed/expressing cells, ultimately inducing apoptosis through the Csp-3 pathway.
Furthermore, several other triterpenes of PE were reported to have anticancer effects on two types of prostate cancer cells, DU145 (androgen-insensitive) and LNCaP (androgen-responsive), and lung cancer A549 cells. Proliferation of both DU145 and LNCaP cells were inhibited presumably through a disruption of the Akt signaling pathway, leading to apoptosis [44]. Interestingly, these triterpenes have better cytotoxicity or anticancer effects on A549 cells than prostate cancer cells [19], implying their cancer-specific or selective anticancer activities.
Besides anticancer activity of PE, its antitumor activity has been also demonstrated elsewhere. In the early study [45], a bioactive polysaccharide, one of PE derivatives, showed its antitumor effect on sarcoma 180 tumor cells grown in mice, inhibiting a > 96% of tumor growth. Several triterpene derivatives of PE also demonstrated their antitumor activities in a two-stage carcinogenesis test on mouse skin using 7, 12-dimethylbenz(a)anthracene (DMBA) as an initiator and 12-O-tetradecanoylphorbol-13-acetate (TPA) as a promoter [46]. The inhibitory effects on skin tumor growth were calculated to be the IC50 of 195 - 340 mol/32 pmol of TPA, showing effective antitumor activities of these PE derivatives.
It is also worthwhile mentioning that anticancer/antitumor activities of polysaccharides of PE have been documented to vary with the strains of Poria mushrooms, monosaccharide composition, protein content, and molecular mass in the studies using mice bearing sarcoma 180 tumors, leukemic HL-60 cells, and liver cancer HepG2 cells [47, 48]. Particularly, derivatives of β-glucan (among polysaccharides) would have different anticancer/antitumor activities, depending upon their structural differences/modifications. Such studies were performed using sarcoma 180 tumor cells in vitro and in vivo and two types of gastric cancer cells, MKN-45 and SGC-7901, in vitro. The results showed that anticancer/antitumor activities were significantly greater in β-glucans with sulfation and carboxymethylation than native β-glucan in vivo as well as in vitro [49]. These findings suggest that interestingly anticancer/antitumor activities of polysaccharides of PE could vary with those different factors described above.
Lastly, it is important to address the “safety” of Poria mushroom because of its potential clinical utility, although it was briefly mentioned earlier. Historically, Poria mushroom has been widely used to treat retention of phlegm and edema, nephrosis, chronic gastritis, poor appetite, loose stool, palpitation, insomnia, etc. [18, 50], over 2,000 years mostly in China. After all these years, it has been also described to have no adverse reactions or side effects in the literature. Actually, Poria mushroom and its various extracts (including PE) [50] are currently used in a number of commercial products, including phytomedicines (for those ailments described above), dietary supplements (for general health maintenance), food or food supplements (soups, cakes, tea, beverages, etc.) and so forth [18]. Based on all available information, it is thus conceivable that Poria mushroom and its extracts are rather safe to be used/consumed in patients as well as normal subjects, although their therapeutic efficacy on certain diseases/disorders yet remains elusive and requires further investigations.
After all, PE may inhibit glycolysis with the modulations of metabolic pathways, induce a cell cycle arrest and ER stress, and ultimately lead to (ER stress-induced) apoptosis. Therefore, it is plausible that targeting ER stress could be a potential alternative strategy for cancer therapy.
Conclusions
In the present study, the Poria mushroom extract, PE, appears to be a promising natural oral agent capable of significantly reducing cell viability and ultimately leading to ER-induced apoptosis in human RCC, ACHN cells. Thus, PE may have potential clinical implications as an adjuvant agent/supplement offering the therapeutic option for RCC, although more studies are yet required.
Acknowledgments
We thank Donna Noonan (Mushroom Wisdom, Inc.) for a gift of PE and also the generous financial support.
Financial Disclosure
This study was funded by Mushroom Wisdom, Inc. (East Rutherford, NJ).
Conflict of Interest
All authors declare no competing interests in this study.
Informed Consent
Not applicable.
Author Contributions
AD (Urology resident) primarily performed experiments. JE (Urology resident) also performed experiments with AD. MC (Department Chair) oversaw the entire project and managed all financial matters. SK (Principal investigator) designed experiments, interpreted the data, and wrote the draft.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
RCC: renal cell carcinoma; PE: Poria mushroom extract; IFN: interferon; IL: interleukin; MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide; HK: hexokinase; AMPK: AMP-activated protein kinase; Akt: protein kinase B; mTOR: mammalian target of rapamycin; ATF4: activating transcription factor 4; CHOP: C/EBP homologous protein; GRP78: glucose-regulated protein 78; Csp-3: caspace-3; PARP: poly-ADP ribose polymerase; ER: endoplasmic reticulum; UPR: unfolded protein response; SD: standard deviation; ANOVA: analysis of variance; IC50: the half maximal (50%) inhibitory concentration
| References | ▴Top |
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354-366.
doi pubmed - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Jacobsohn KM, Wood CG. Adjuvant therapy for renal cell carcinoma. Semin Oncol. 2006;33(5):576-582.
doi pubmed - Mori K, Mostafaei H, Miura N, Karakiewicz PI, Luzzago S, Schmidinger M, Bruchbacher A, et al. Systemic therapy for metastatic renal cell carcinoma in the first-line setting: a systematic review and network meta-analysis. Cancer Immunol Immunother. 2021;70(2):265-273.
doi pubmed - Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477-2490.
doi pubmed - Malkoc E, Ramirez D, Kara O, Maurice MJ, Nelson RJ, Caputo PA, Kaouk JH. Robotic and open partial nephrectomy for localized renal tumors larger than 7 cm: a single-center experience. World J Urol. 2017;35(5):781-787.
doi pubmed - Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36(12):1943-1952.
doi pubmed - Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163(2):408-417.
doi - Margulis V, McDonald M, Tamboli P, Swanson DA, Wood CG. Predictors of oncological outcome after resection of locally recurrent renal cell carcinoma. J Urol. 2009;181(5):2044-2051.
doi pubmed - Glaspy JA. Therapeutic options in the management of renal cell carcinoma. Semin Oncol. 2002;29(3 Suppl 7):41-46.
doi pubmed - Gitlitz BJ, Figlin RA. Cytokine-based therapy for metastatic renal cell cancer. Urol Clin North Am. 2003;30(3):589-600.
doi - Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr., et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655-1659.
doi pubmed - Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7(5):277-285.
doi pubmed - Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633-4646.
doi pubmed - Simons AL, Mattson DM, Dornfeld K, Spitz DR. Glucose deprivation-induced metabolic oxidative stress and cancer therapy. J Cancer Res Ther. 2009;5(Suppl 1):S2-6.
doi pubmed - Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
doi pubmed - Jang M, Kim SS, Lee J. Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med. 2013;45:e45.
doi pubmed - Rios JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011;77(7):681-691.
doi pubmed - Zhou L, Zhang Y, Gapter LA, Ling H, Agarwal R, Ng KY. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem Pharm Bull (Tokyo). 2008;56(10):1459-1462.
doi pubmed - Chen X, Zhang L, Cheung PC. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1→3)-D-glucan from Poria cocos. Int Immunopharmacol. 2010;10(4):398-405.
doi pubmed - Zhao YY, Lei P, Chen DQ, Feng YL, Bai X. Renal metabolic profiling of early renal injury and renoprotective effects of Poria cocos epidermis using UPLC Q-TOF/HSMS/MSE. J Pharm Biomed Anal. 2013;81-82:202-209.
doi pubmed - Miccoli L, Oudard S, Sureau F, Poirson F, Dutrillaux B, Poupon MF. Intracellular pH governs the subcellular distribution of hexokinase in a glioma cell line. Biochem J. 1996;313(Pt 3):957-962.
doi pubmed - Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, et al. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10(12):2350-2362.
doi pubmed - Priebe A, Tan L, Wahl H, Kueck A, He G, Kwok R, Opipari A, et al. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol. 2011;122(2):389-395.
doi pubmed - Lee YK, Park OJ. Regulation of mutual inhibitory activities between AMPK and Akt with quercetin in MCF-7 breast cancer cells. Oncol Rep. 2010;24(6):1493-1497.
doi - Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977-23980.
doi pubmed - Loar P, Wahl H, Kshirsagar M, Gossner G, Griffith K, Liu JR. Inhibition of glycolysis enhances cisplatin-induced apoptosis in ovarian cancer cells. Am J Obstet Gynecol. 2010;202(4):371.e371-378.
doi pubmed - Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60(14):3689-3695.
- Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97(23):12625-12630.
doi pubmed - Wu Z, Wang H, Fang S, Xu C. Roles of endoplasmic reticulum stress and autophagy on H2O2induced oxidative stress injury in HepG2 cells. Mol Med Rep. 2018;18(5):4163-4174.
doi - Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23(2):143-149.
doi pubmed - Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066-3077.
doi pubmed - Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14(4):263-276.
doi pubmed - Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173-194.
doi pubmed - Yang Q, Li S, Fu Z, Lin B, Zhou Z, Wang Z, Hua Y, et al. Shikonin promotes adriamycininduced apoptosis by upregulating caspase3 and caspase8 in osteosarcoma. Mol Med Rep. 2017;16(2):1347-1352.
doi pubmed - Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7(4):321-328.
doi pubmed - Zhang M, Chiu LC, Cheung PC, Ooi VE. Growth-inhibitory effects of a beta-glucan from the mycelium of Poria cocos on human breast carcinoma MCF-7 cells: cell-cycle arrest and apoptosis induction. Oncol Rep. 2006;15(3):637-643.
doi - Chen YY, Chang HM. Antiproliferative and differentiating effects of polysaccharide fraction from fu-ling (Poria cocos) on human leukemic U937 and HL-60 cells. Food Chem Toxicol. 2004;42(5):759-769.
doi pubmed - Mizushina Y, Akihisa T, Ukiya M, Murakami C, Kuriyama I, Xu X, Yoshida H, et al. A novel DNA topoisomerase inhibitor: dehydroebriconic acid, one of the lanostane-type triterpene acids from Poria cocos. Cancer Sci. 2004;95(4):354-360.
doi pubmed - Zoni E, Chen L, Karkampouna S, Granchi Z, Verhoef EI, La Manna F, Kelber J, et al. CRIPTO and its signaling partner GRP78 drive the metastatic phenotype in human osteotropic prostate cancer. Oncogene. 2017;36(33):4739-4749.
doi pubmed - Li Z, Li Z. Glucose regulated protein 78: a critical link between tumor microenvironment and cancer hallmarks. Biochim Biophys Acta. 2012;1826(1):13-22.
doi pubmed - Xia S, Duan W, Liu W, Zhang X, Wang Q. GRP78 in lung cancer. J Transl Med. 2021;19(1):118.
doi pubmed - Kang HM, Lee SK, Shin DS, Lee MY, Han DC, Baek NI, Son KH, et al. Dehydrotrametenolic acid selectively inhibits the growth of H-ras transformed rat2 cells and induces apoptosis through caspase-3 pathway. Life Sci. 2006;78(6):607-613.
doi pubmed - Gapter L, Wang Z, Glinski J, Ng KY. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun. 2005;332(4):1153-1161.
doi pubmed - Kanayama H, Adachi N, Togami M. A new antitumor polysaccharide from the mycelia of Poria cocos wolf. Chem Pharm Bull (Tokyo). 1983;31(3):1115-1118.
doi pubmed - Akihisa T, Nakamura Y, Tokuda H, Uchiyama E, Suzuki T, Kimura Y, Uchikura K, et al. Triterpene acids from Poria cocos and their anti-tumor-promoting effects. J Nat Prod. 2007;70(6):948-953.
doi pubmed - Jin Y, Zhang L, Zhang M, Chen L, Cheung PC, Oi VE, Lin Y. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohydr Res. 2003;338(14):1517-1521.
doi - Zhang L, Chen L, Xu X, Zeng F, Cheung PC. Effect of molecular mass on antitumor activity of heteropolysaccharide from Poria cocos. Biosci Biotechnol Biochem. 2005;69(3):631-634.
doi pubmed - Wang Y, Zhang L, Li Y, Hou X, Zeng F. Correlation of structure to antitumor activities of five derivatives of a beta-glucan from Poria cocos sclerotium. Carbohydr Res. 2004;339(15):2567-2574.
doi pubmed - Jia X, Ma L, Li P, Chen M, He C. Prospects of Poria cocos polysaccharides: isolation process, structural features and bioactivities. Trends Food Sci Technol. 2016;54:52-62.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
