| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Case Report
Volume 12, Number 1, July 2023, pages 17-21
Greens Mean Go: A Case Report Exploring a Vegetarian Diet in Chronic Kidney Disease
Qwynton Johnsona, b, e, Alexis Kirkb, Sydney Sykesc, Andrea Asiedud, Sundeep Shahd
aKansas City University, Kansas City, MO, USA
bCollege of Osteopathic Medicine, Kansas City University, Kansas City, MO, USA
cVirginia Commonwealth University Bon Secours St. Francis Family Medicine, Midlothian, VA, USA
dPremier Medical Associates, The Villages, FL, USA
eCorresponding Author: Qwynton Johnson, Kansas City University, Kansas City, MO, USA
Manuscript submitted May 18, 2023, accepted June 2, 2023, published online July 12, 2023
Short title: Vegetarian Diet in Chronic Kidney Disease
doi: https://doi.org/10.14740/wjnu443
| Abstract | ▴Top |
An 81-year-old Caucasian male with stage 4 chronic kidney disease (CKD) successfully avoided dialysis through specific lifestyle modifications. By following a strict vegetarian diet, avoiding animal proteins entirely, and adhering to a medication regimen, there was an immediate improvement in his kidney function. Three years into his newly adopted lifestyle, he remains off dialysis. This case report discusses the complex relationship between diet and chronic kidney disease.
Keywords: Chronic kidney disease; Vegetarian; Creatinine; Glomerular filtration rate; Diet; Dialysis; Ammonia genesis; Acid-base; Urea; Metabolism
| Introduction | ▴Top |
Chronic kidney disease (CKD) is a worldwide health concern affecting millions annually. The estimated global prevalence of CKD is 13.4%, with as many as 7 million individuals requiring renal replacement therapy [1]. Traditionally, CKD is defined as having a glomerular filtration rate (GFR) of less than 60 mL/min or evidence of kidney damage reflected by changes in blood tests, imaging, or biopsies for at least 3 months. Furthermore, patients are stratified into stages of CKD depending on the severity [2].
When an individual is classified as having stage 5 CKD, they are considered to have end-stage renal disease (ESRD), and the patient will require renal replacement therapy. Renal replacement therapy is achieved through dialysis with or without a kidney transplant. The most effective therapy to delay the progression of CKD is widely debated among nephrologists. However, the provider’s primary concern for their patient remains to avoid ESRD and a need for renal replacement therapy.
Medications and dietary changes have been at the forefront of CKD treatment. Avoiding protein consumption has been a primary focus in delaying the progression of kidney disease. Protein is ubiquitous in various diets but is considerably more concentrated in animal products. Thus, patients with CKD are educated to abstain from consuming animal proteins. Protein metabolism induces a temporary hyperfiltration rate of the kidneys, which has been theorized to accelerate disease progression and worsen renal function [3]. Additionally, a protein-rich diet increases dietary acid load and other compounds that lead to trimethylamine-N-oxide formation, potentially worsening kidney disease [4].
Specific diets have been proposed as being beneficial in delaying the progression of kidney disease, specifically vegetarian and vegan/plant-based diets. Plant-derived proteins are digested differently than animal-based proteins. Additionally, plant-based diets have multiple benefits, such as increased fiber intake, which helps with digestion and regulating bowel habits. Preventing constipation promotes the excretion of uric acid, which is toxic when accumulated in significant amounts [5]. Numerous trials have studied the effects of low-protein diets on the progression of CKD. These studies are limited due to sample size and the need for clear delineation between animal and plant-based proteins [6].
The function of the kidney is to remove byproducts of protein metabolism and other toxic metabolites. When the kidneys are damaged, and the filtration quality is impaired, it results in the buildup of toxic waste products. Under these conditions, patients can become uremic, clinically presenting with anorexia, nausea/vomiting, fatigue, confusion, and other nonspecific symptoms. When this occurs, immediate renal replacement therapy in the form of dialysis is required.
| Case Report | ▴Top |
This is an 81-year-old Caucasian male with a medical history of stage 4 CKD, hypertension, hyperlipidemia, hyperuricemia, and paroxysmal atrial fibrillation. His medication regimen includes Eliquis, furosemide, carvedilol, allopurinol, pantoprazole, isosorbide mononitrate, and sodium bicarbonate. He has a prominent cardiac history of coronary artery bypass graft surgery and pacemaker placement. The patient is physically active and does not endorse tobacco or alcohol use.
Multiple nephrologists evaluated the patient over decades for the gradual deterioration of his kidney function. His hemoglobin A1c and fasting glucose have consistently been within the non-diabetic range. Renal ultrasound failed to demonstrate any anatomic abnormalities. The patient has no significant family history of renal disease, and other common causes of kidney disease were excluded. He was diagnosed with hypertensive nephropathy due to uncontrolled hypertension for the past 40 years. He has never undergone a kidney biopsy.
His kidney function continued to decline steadily, and dialysis treatment was imminent. In the spring of 2019, the patient had a peritoneal catheter placed, and peritoneal dialysis (PD) education began. Amid dialysis training, a 2-L fill volume was not tolerated due to an inguinal hernia causing leakage of peritoneal fluid into the scrotum. The patient was not motivated to continue dialysis due to intolerance and his abdominal wall defect. The hernia was eventually repaired, and ultimately PD cycler was not started.
The patient started researching various online sources on alternative methods for improving kidney function. After a discussion with his physician, they agreed to trial lifestyle changes before initiating dialysis. His lifestyle modification included adopting a strict vegetarian diet and refraining from all animal proteins. At this time, the patient was prescribed sodium bicarbonate 325 mg twice per day. After weeks of staying disciplined with his medication regimen and vegetarian diet, his bloodwork reflected an increase in his overall kidney function. His estimated glomerular filtration rate (eGFR) increased from 13 to 18 mL/min, while his creatinine decreased from 4.23 to 3.13 mg/dL. Additionally, in 4 months, his blood urea nitrogen (BUN) decreased from 85 to 54 mg/dL (Table 1, Figs. 1-3). With the serum creatinine gradually stabilizing, dialysis was no longer necessary, and the peritoneal catheter was removed.
 Click to view | Table 1. Kidney Function Represented Through Creatinine, eGFR, and BUN |
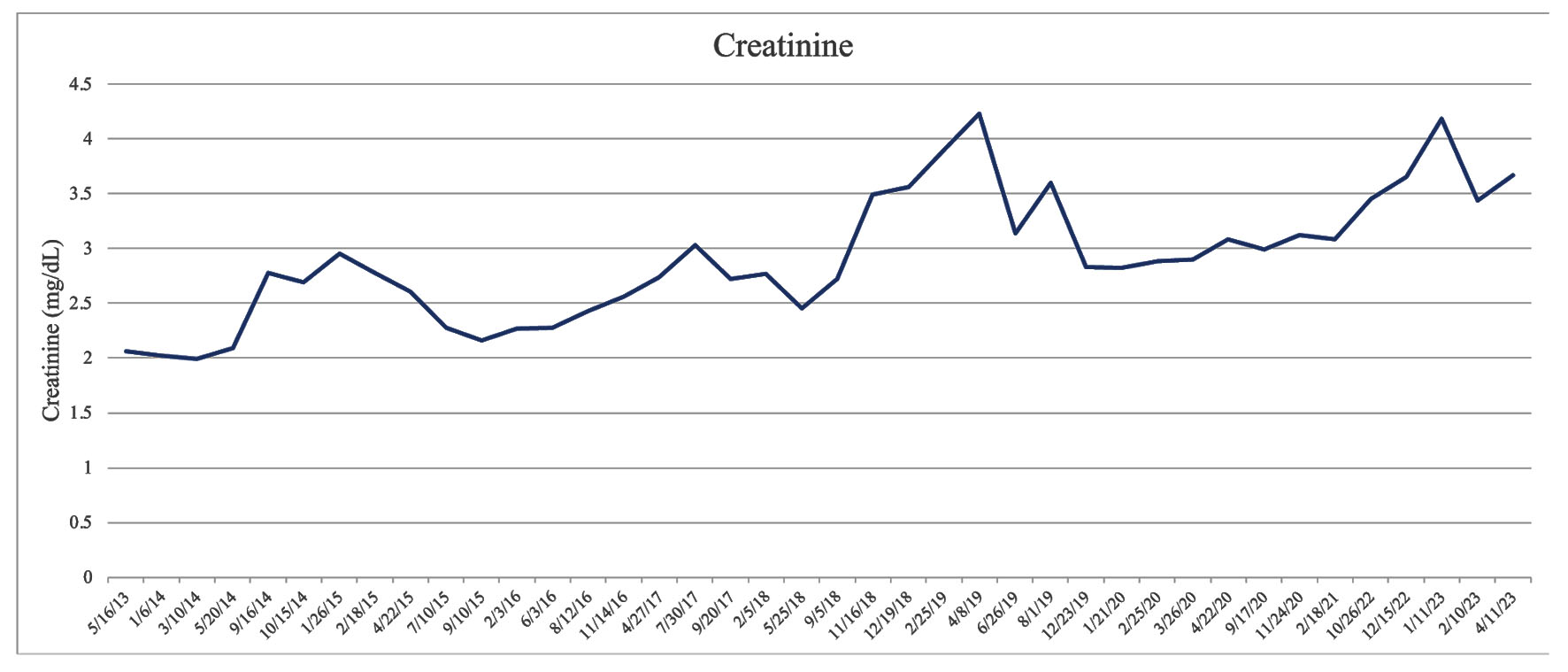 Click for large image | Figure 1. Kidney function represented through creatinine. |
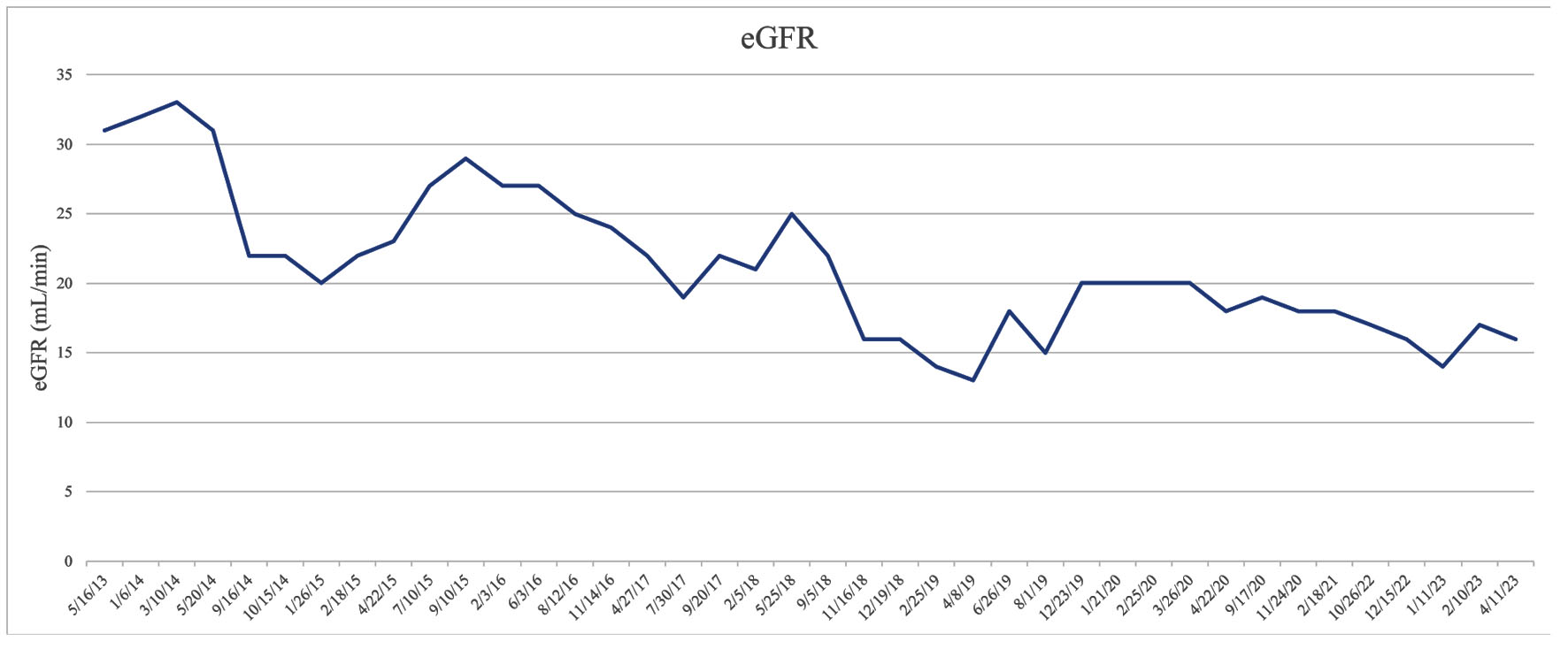 Click for large image | Figure 2. Kidney function represented through eGFR. eGFR: estimated glomerular filtration rate. |
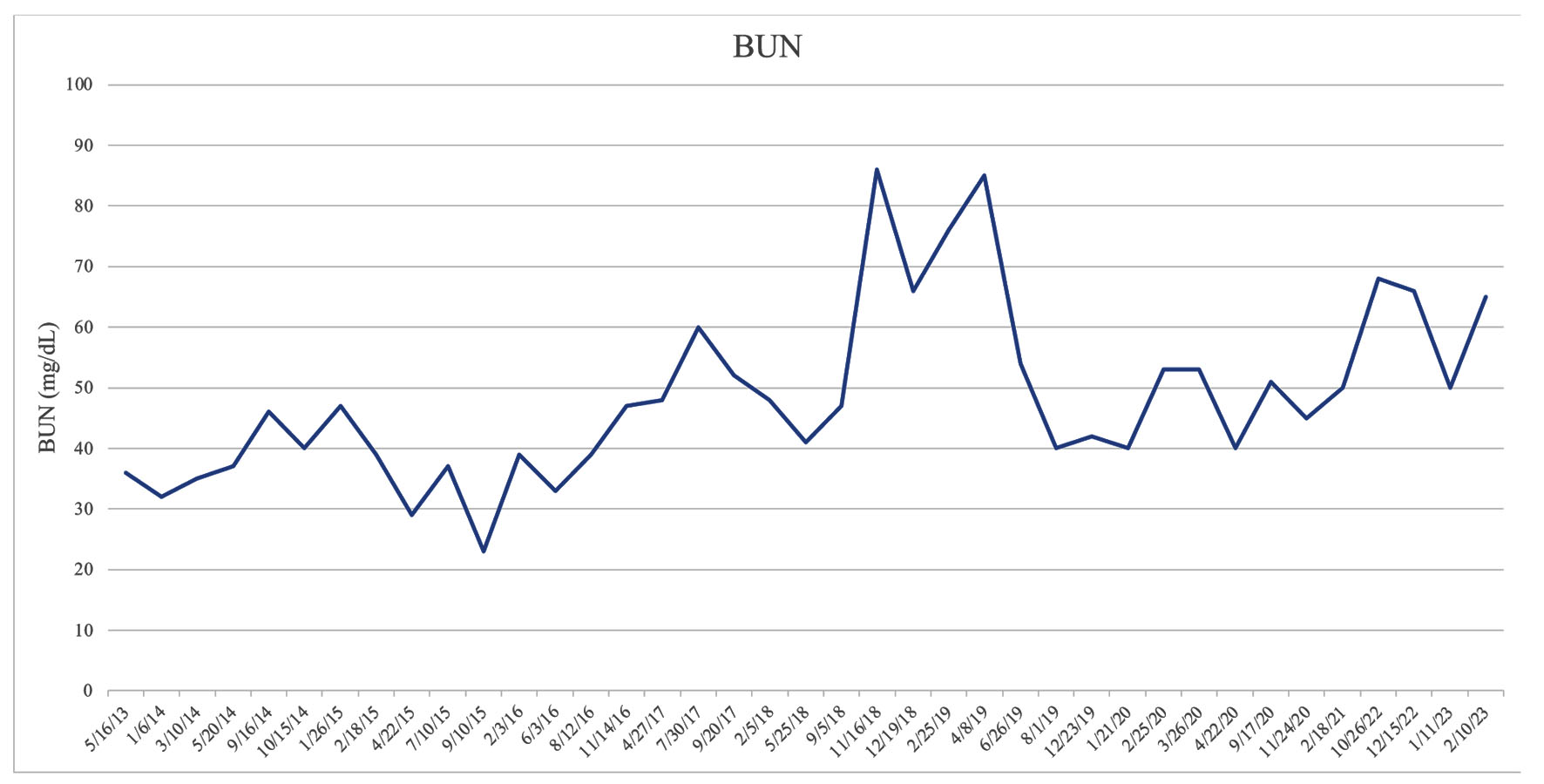 Click for large image | Figure 3. Kidney function represented through BUN. BUN: blood urea nitrogen. |
The patient adequately controls his hypertension with a regimen of furosemide and carvedilol (Table 2). He continues religiously following his vegetarian diet and has remained off dialysis for 3 years. This 3-year interval has been complicated by three spontaneous gastrointestinal bleeds (September 2021, September 2022, and January 2023), likely due to the anticoagulation he is taking for atrial fibrillation. The acute insult to his CKD momentarily impairs his kidney function. However, with his medications, lifestyle modifications and the resilience of his kidneys, his renal function promptly returns to his baseline GFR of 17 - 18 mL/min.
 Click to view | Table 2. Blood Pressure Log |
| Discussion | ▴Top |
Urea production
The kidneys are vital in ammonia genesis and urea metabolism, and essential for overall acid-base homeostasis and eliminating nitrogenous waste. As proteins are degraded into amino acids, they can be further processed by the liver to form urea or used in protein synthesis [7]. There is a direct correlation between the amount of protein consumed to the amount of urea produced. The quantity of urea in an individual can be measured by a blood urea nitrogen (BUN) test, which is extrapolated from a blood sample. GFR and BUN are inversely proportional; as GFR decreases, as seen in conditions such as CKD, the BUN or retained urea content increases [8]. This relationship is apparent with a heavy animal protein diet. BUN levels accumulate when the kidneys cannot metabolize urea and nitrogenous waste products, and consequently, a uremic state can ensue. When our patient started his vegetarian diet and refrained from consuming animal proteins, his BUN decreased significantly from 85 to 54 mg/dL.
Ammonia production
The complex interplay of ammonium metabolism in the kidney serves many purposes, including acid-base homeostasis and ammonium disposal. Ammonia genesis plays an essential role in acid-base homeostasis, which often becomes jeopardized when suffering from CKD. When the kidney’s functional units become damaged, their ability to generate ammonia will be compromised. When the ability to generate ammonia in the proximal convoluted tubule (PCT) is impaired, acid excretion and bicarbonate generation will fall. When these biochemical processes become deranged, metabolic acidosis will ensue. Another integral function is the disposal of ammonium. Ammonium is water-soluble and is excreted in urine. In contrast, its ammonia counterpart is lipid-soluble, which cannot be eliminated. If ammonia accumulates in large amounts, it can cross the blood-brain barrier and have toxic effects on the brain and other organs.
Acid production
An animal protein diet results in a sizable dietary acid load due to the breakdown of sulfur- containing amino acids such as methionine and cysteine. In meat and eggs alone, sulfur- containing amino acids can be two to five times greater than in legumes and grains [9]. With the increased dietary acid load after animal protein consumption, shifts in acid-base homeostasis are necessary. Without correction, long-standing metabolic acidosis can have a significant impact on overall health, including bone mineral disease, muscle breakdown, and potentially even renal fibrosis [10]. To correct the acid-base imbalances introduced by animal protein consumption, current guidelines recommend prescribing alkali therapy in the form of sodium bicarbonate [10]. The recommendation is to maintain the serum sodium bicarbonate level at a minimum of 22 mEq/L, with ongoing research dedicated to the benefits of increasing this minimum to obtain better control [2]. When there is better control over acid-base homeostasis, there is less demand on the kidneys to produce increasing amounts of ammonia, which in turn lessens the stress on the kidneys. Studies have shown that supplementation of alkali therapy both slows the rate of GFR decline and delays the progression to ESRD [10].
Learning points
Individuals with CKD are often prescribed medications and encouraged to make dietary changes before starting renal replacement therapy. In this case report, an 81-year-old Caucasian male with stage 4 CKD wished to defer dialysis and attempt lifestyle modifications in addition to a guideline-based pharmacological regimen. Removing all animal proteins, consuming a vegetarian diet, and pharmacological compliance improved his kidney function, and dialysis treatment was no longer necessary. As discussed above, animal proteins adversely affect kidneys through various processes. Consumption of animal-derived proteins increases dietary acid load and urea production. The kidneys are responsible for ammonia genesis and other biochemical pathways maintaining acid-base homeostasis. When the kidneys are damaged, as seen in CKD, there is a reduction in functional nephrons. The increased demand for ammonia genesis and excretion of acid can overload the remaining nephrons, ultimately accelerating kidney damage and the progression of CKD. Animal protein consumption can also increase pressures within the glomerulus, leading to hyperfiltration, further damaging the kidneys. After thorough consideration, the patient elected to embrace a vegetarian diet. The patient regained kidney function, as reflected by an increase in GFR and a decrease in BUN. By removing animal protein from their diet and staying compliant to their medications, the patient could delay the need for dialysis and improve their overall kidney function.
This report is not suggesting that the cessation of animal protein as the only intervention will reverse kidney damage. The benefit of a vegetarian diet supplementing a guideline-based medication regimen proved helpful for this particular individual. As researchers in a community setting, our resources are limited. We hope that larger institutions will explore this idea and perform more extensive studies examining the independent benefits of vegetarian diets with or without bicarbonate supplementation on CKD.
Acknowledgments
We want to acknowledge the patient’s willingness to contribute to medical science. Additionally, we would like to thank Dr. Sundeep Shah, MD FASN, for his insight and guidance throughout this report.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Study conception and design: Sundeep Shah MD FASN; data collection: Andrea Asiedu MD; interpretation of results: Sydney Sykes MD; draft manuscript preparation: Qwynton Johnson, Alexis Kirk. All authors reviewed the results and approved the final version of the manuscript.
Data Availability
The data supporting this study’s findings are openly available on scholarly peer-reviewed journal databases.
Abbreviations
CKD: chronic kidney disease; GFR: glomerular filtration rate; ESRD: end-stage renal disease; PD: peritoneal dialysis; BUN: blood urea nitrogen
| References | ▴Top |
- Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3-15.
doi pubmed - Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238-1252.
doi pubmed - Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab (Lond). 2005;2:25.
doi pubmed pmc - Joshi S, McMacken M, Kalantar-Zadeh K. Plant-based diets for kidney disease: a guide for clinicians. Am J Kidney Dis. 2021;77(2):287-296.
doi pubmed - Hosomi A, Nakanishi T, Fujita T, Tamai I. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PLoS One. 2012;7(2):e30456.
doi pubmed pmc - Murdeshwar HN, Anjum F. Hemodialysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
pubmed - Osuna-Padilla IA, Leal-Escobar G, Garza-Garcia CA, Rodriguez-Castellanos FE. Dietary Acid Load: mechanisms and evidence of its health repercussions. Nefrologia (Engl Ed). 2019;39(4):343-354.
doi pubmed - Mulroney Susan, et al. Regulation of acid-base balance by the kidneys. Netter’s Essential Physiology, 2nd ed. Elsevier, Philadelphia, PA. 2016. p. 237-247
- Hamidianshirazi M, Ekramzadeh M. Dietary acid load and chronic kidney disease. Saudi J Kidney Dis Transpl. 2021;32(6):1511-1522.
doi pubmed - de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9):2075-2084.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
