| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Original Article
Volume 13, Number 1, July 2024, pages 19-25
Serum Cystatin C as an Index of Early Detection of Acute Kidney Injury in Children With Severe Malaria
Korede O. Oluwatuyia, Abel O. Alongea, Ifedayo O. Fasorantia, Tolulope O. Jegedea, Qasim O. Salaua, Abiodun J. Kareema, e, Olajumoke M. Aiyekua, Foluwakemi T. Ekogiawea, Opeyemi O. Akinmadeloa, Grace Adetonaa, Adebowale D. Ademolab, Adanze O. Asinobib, Sikiru Biliaminuc, Osahon J. Asowatad
aDepartment of Paediatrics, Federal Medical Centre, Owo, Ondo State, Nigeria
bDepartment of Paediatrics, University College Hospital, Ibadan, Oyo State, Nigeria
cDepartment of Chemical Pathology and Immunology, College of Health Sciences, University of Ilorin, Ilorin, Kwara State, Nigeria
dDepartment of Epidemiology and Medical Statistics, College of Medicine University of Ibadan, Oyo State, Nigeria
eCorresponding Author: Abiodun John Kareem, Department of Paediatrics, Federal Medical Centre, Owo, Ondo State, Nigeria
Manuscript submitted August 19, 2023, accepted September 18, 2023, published online July 5, 2024
Short title: Cystatin C as an Indicator of AKI
doi: https://doi.org/10.14740/wjnu445
| Abstract | ▴Top |
Background: Acute kidney injury (AKI) in severe malaria is a major cause of morbidity and mortality. It is often diagnosed using serum creatinine (Scr), which has several limitations. A test for early confirmation of AKI devoid of these limitations is therefore required. Serum cystatin C is therefore suggested as an early screening tool in diagnosing AKI in severe malaria. This study aimed at determining the accuracy of serum cystatin C in the early diagnosis of AKI in severe malaria.
Methods: This was a cross-sectional study. Children with World Health Organization (WHO)-defined severe malaria were recruited and AKI was defined using the Kidney Disease: Improving Global Outcomes (KDIGO) and WHO criteria as well as a serum cystatin C level of > 0.95 mg/L. The Scr and cystatin C levels were done and data were analyzed with a P-value less than 5% considered significant.
Results: A total of 126 children aged 1 - 15 years comprising 70 (55.6%) males with a male-to-female ratio of 1.25:1 were studied. The prevalence of AKI was 38.9% using serum cystatin C, 23.8% using KDIGO and 11.9% using WHO criteria with statistically significant difference (P = 0.001). The mean serum cystatin C was 0.74 (0.59) mg/L. The sensitivity, specificity, and negative predictive value of cystatin C in the diagnosis of AKI in severe malaria were 80.0%, 73.9%, and 92.2%, respectively using the KDIGO definition of AKI.
Conclusion: Serum cystatin C has a high sensitivity, specificity and negative predictive value and can be used to screen children for early detection of AKI in severe malaria.
Keywords: Acute kidney injury; Children; Cystatin C; Malaria
| Introduction | ▴Top |
Severe malaria is a life-threatening parasitic infection of public health importance, present in about 91 countries of the world and endemic in sub-Saharan Africa including Nigeria [1]. Plasmodium falciparum is the most common malaria parasite species accounting for 99.7% of estimated malaria cases and is notorious for causing complications and death [1, 2]. In 2021, the World Health Organization (WHO) estimated that 245 million cases of malaria occurred worldwide, of which 94% occurred in African, while Nigeria accounted for the highest prevalence of 27% of the global cases [1, 3]. Furthermore, Africa accounted for 94% of the total 619,000 malaria deaths globally and the most vulnerable age groups were children under 5 years who accounted for 76% of these deaths [3]. In the same vein, about a quarter of these global deaths occurred in Nigeria [1]. These deaths are mainly from the complications of severe form of P. falciparum malaria. These complications include cerebral malaria, severe anemia, hypoglycemia, hemoglobinuria and acute kidney injury (AKI) [4].
The prevalence of AKI has been on the increase contributing to 45% of mortality in patients with severe malaria [5-7]. AKI is defined as sudden deterioration in kidney function with accumulation of nitrogenous waste and creatinine [8]. The Kidney Disease: Improving Global Outcomes (KDIGO) defines AKI as an increase in serum creatinine (Scr) by at least 0.3 mg/dL (26.5 µmol/L) occurring within 48 h of admission or increase in Scr levels above 1.5 times of baseline value which is known or presumed to have occurred within 7 days or urine output less than 0.5 mL/kg/h for 6 h [9].
The Scr is the most widely available endogenous marker of kidney function utilized in most studies to detect AKI in severe malaria [10, 11]. The WHO defines AKI in severe malaria as Scr level of ≥ 3 mg/dL and adequate volume repletion; however, it has been observed that at Scr of 3 mg/dL, most patients would have been in established AKI using the KDIGO criteria with its possible numerous untoward effects [9].
The Scr may not be an early marker for AKI because of its drawbacks which include the effect of age, sex, diet, and muscle bulk on its value [12]. The Scr level is also affected by its tubular secretions during renal injury, its delay in rise of serum level following AKI as well as the need for a baseline value which is almost always unavailable in our setting. Other drawbacks include the interference of some substances like bilirubin with its laboratory measurement and it is insensitive to mild to moderate reduction in glomerular filtration rate (GFR) [13]. The shortfalls of Scr necessitated the search for a better biomarker of AKI that predicts early development of AKI for prompt institution of appropriate therapy and prognostication.
Cystatin C is one of the novel, relatively cheap and readily available biomarkers of AKI that is devoid of the various challenges experienced with Scr. There is however paucity of data to demonstrate its use in children with severe malaria. Therefore, this study was carried out to determine the usefulness of cystatin C in the early detection of AKI in children with severe malaria. The objective was to determine the value of cystatin C in the early diagnosis of AKI in severe malaria. It is envisaged that the proposed study will identify children with AKI in severe malaria that would have been missed with the use of Scr-based KDIGO and WHO definitions.
| Materials and Methods | ▴Top |
Study site
The study was carried out at the Federal Medical Centre (FMC), Owo. The hospital serves as a referral center to hospitals within and outside Owo such as Edo, Ekiti and Kogi States. The town lies on latitude 7°11' of the equator and longitude 50°35' of the Greenwich meridian [14, 15]. The population of Owo Local Government Area (LGA) is 218,886 [16]. The Children Emergency Ward (CHEW) of FMC, Owo is a 20-bedded ward with four consulting rooms. It is manned by medical doctors (consultant pediatricians, senior registrars, registrars, and medical interns) assisted by several nurses. The CHEW is the points of entry of all patients admitted to the Department of Pediatrics and it receives a range of 10 - 15 admissions per day.
Study design
The study was a cross-sectional study.
Subjects recruitment
This study was conducted among children aged between 1 and 15 years who presented to the CHEW of FMC, Owo and met the WHO clinical and/or laboratory criteria for severe malaria [1]. Children with established/background renal disease, subjects with conditions that can affect the level of cystatin C like human immunodeficiency virus (HIV) infection, malnutrition, thyroid pathology, and steroid therapy were excluded from the study.
Sample size
The minimum sample size was calculated using the formula below [17]:
Allowing for attrition of 10%, the minimum sample size is 126 participants.
Sampling technique
All consecutive admissions into the CHEW whose ages were from 1 to 15 years who met the inclusion criteria for severe malaria were recruited. They were tested for the presence of asexual form of P. falciparum using microscopy method. Those that tested positive for the asexual form of P. falciparum and whose parents had given consent were recruited into the study until the sample size was completed.
Ethical approval
The study was approved by the Ethical Review Committee of the Federal Medical Centre, Owo with number FMC/OW/380/VOL.CXXXVI/95. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Study period
The study was conducted from November 2020 to September 2021.
Study procedure
All consecutive admissions into the CHEW who met the inclusion criteria during the period of the study were recruited. Data were collected in a proforma which included history, examination and laboratory investigations. The information obtained included age, sex, renal symptoms and clinical presentations. The socio-economic class was determined using Ogunlesi classification of social class [19]. This was calculated based on the level of education and occupation of both parents. Scores (ranging from 1 to 5) were assigned to the educational qualification and occupation of the father and the mother. The average of these four scores, to the nearest whole number, constituted the social class of the subject. A thorough general physical and systemic examination was carried out on all subjects recruited looking out for features of severe malaria that were not captured in the history. Also, features suggestive of a kidney injury such as body swelling were sought for.
Sample collection and laboratory investigation
At admission, serum electrolyte, urea, creatinine, blood film for malaria parasite, HIV antibody and cystatin C were measured. A provider-initiated pre-testing counselling was done before retroviral screening test. This was done to exclude HIV which is one of the established factors that affect serum cystatin C level.
The thin and thick film smears were both prepared from drops of blood obtained directly on a pre-cleaned slide at the point of recruiting the patients. The thick films were used to screen blood for the presence of trophozoites of Plasmodium, while the thin films were used for parasite species identification.
At 36 h after admission, another 1 mL of blood was collected for a repeat Scr estimation. The aliquot of samples was allowed to clot then centrifuged at 3,000 revolutions per minute for 5 min in a bench-top centrifuge to extract the serum. The serum obtained from the blood was analyzed subsequently for Scr, urea and electrolyte (potassium and bicarbonate).
The Scr at admission and at 36 h of admission were estimated using the Jaffe’s picric kinetic method [20] using the creatinine kit (CR 510) from RANDOX Laboratories (Ireland).
The serum cystatin C assay was performed using the particle-enhanced turbidimetric immunoassay (PETIA) cystatin C test kit [20] from Centronic GmbH, Germany (August 2018 edition). According to the manufacturer, the reference range for normal serum cystatin C was 0.51 - 0.95 mg/L; therefore, the analytical upper limit of detection for cystatin C was 0.95 mg/L which fell within the finding of Esezobor et al [21]. This informed the reference range of 0.51 - 0.95 mg/L used for this study. Samples were analyzed in batches of 50 samples on Rayto spectrophotometer by Rayto Life and Analytical Sciences Co., Ltd. The procedure employed the use of six bottles labelled standard and test tubes labelled serum. With the use of a pipette, 3 µL of each standard solution was introduced into the six bottles labelled standard. At the same time, 3 µL of serum was introduced in the test tube labelled serum. Nine hundred microliters (900 µL) of reagent 1 (buffer) was then added to all the test tubes labelled serum and standard. This was then mixed and incubated for 1 min.
After incubation, 160 µL of latex reagent 2 was then added to all bottles, incubated for another 5 min and the absorbance was measured at the wavelength of 600 nm in a cuvette. Two readings at 1 min (A1) and at 5 min (A2) were taken for all the 126 samples and the prepared standards. The difference between the two readings was taken and used to determine the concentration of cystatin C on the known standard curve. A standard curve was constructed by plotting absorbance values for the standards against the concentration that was provided with the kit. The concentration of the unknown samples was then determined from the curve. The percentage coefficients of variation (CV) for intra- and inter-serum cystatin C assay were 3.7% and 4.3%, respectively.
Urinary output was monitored over 24 h to determine those who had oliguria/anuria by placing a penile conduit around the penile shaft and an adhesive urine bag attached to the female perineum with a diaper used to hold the bag firmly to avoid leakages. The urine volume was measured in a calibrated urine jar and then recorded. Five milliliters of urine was collected at admission into a universal bottle which was subsequently subjected to urinalysis using a Combi-9 dipstick (Mission® Expert 11UW by ACON).
AKI definition in the study
The KDIGO definition of AKI in this study was any subject with a rise in Scr of at least 0.3 mg/dL between the admitting Scr and that obtained at 36 h of admission as well as urine output less than 0.5 mL/kg/h lasting 6 - 12 h of admission [9]. Subjects with Scr level greater than 3 mg/dL (> 265 µmol/L) and/or 24-h urine output less than 12 mL/kg were considered to have WHO-defined AKI. Serum level of cystatin C greater than 0.95 mg/L was considered to have AKI, according to the manufacturer of Centronic GmbH, Germany (August 2018 edition).
Data analysis
Statistical analysis was done using the Statistical Package for Social Science (SPSS) version 22.0 statistical software. Continuous variables such as age, Scr and serum cystatin C were summarized using mean and standard deviation (SD). Categorical variables such as sex, age group, and presence of AKI were summarized using frequencies, proportions and presented using tables and charts. Independent sample t-test was used to compare means of two normally distributed variables. Sensitivity, specificity, positive predictive value (PPV), and the negative predictive value (NPV) were calculated for WHO, KDIGO and serum cystatin C criteria.
| Results | ▴Top |
During the study period, a total of 521 children were admitted into the CHEW, of whom 159 (30.5%) had severe malaria, of whom 126 (79.2%) subjects who met the inclusion criteria were recruited for the study and 33 (20.8%) were excluded. Of the 33 subjects that were excluded, parents of 14 did not give consent, eight had Lassa fever, while 11 were malnourished. The socio-demographic characteristics of the participants is shown in Table 1. The majority, 79 (62.7%) were under 5 years of age. The number of children in the age group was comparable across the gender strata. There were 70 (55.6%) males with a median age of 3.17 years (interquartile range (IQR): 2.08 - 5.54). Eighty-seven (69.0%) subjects were of Yoruba ethnicity and parents of 52 (41.3%) of the children were from socio-economic class IV. No parent was from socio-economic class I. The majority (76.2%) had used water-based herbal mixture.
 Click to view | Table 1. Socio-Demographic Characteristics of the Study Population |
Forms of severe malaria found in the study population
The WHO severity criteria of malaria found in the recruited children are represented in Figure 1. Severe anemia (89, 70.6%), prostration (77, 61.1%), multiple convulsions (52, 41.3%), and cerebral malaria (31.7%) were the most predominant forms, while the least frequent was hypoglycemia (10.3%). WHO-defined AKI was seen in 15 (11.9%) of the children.
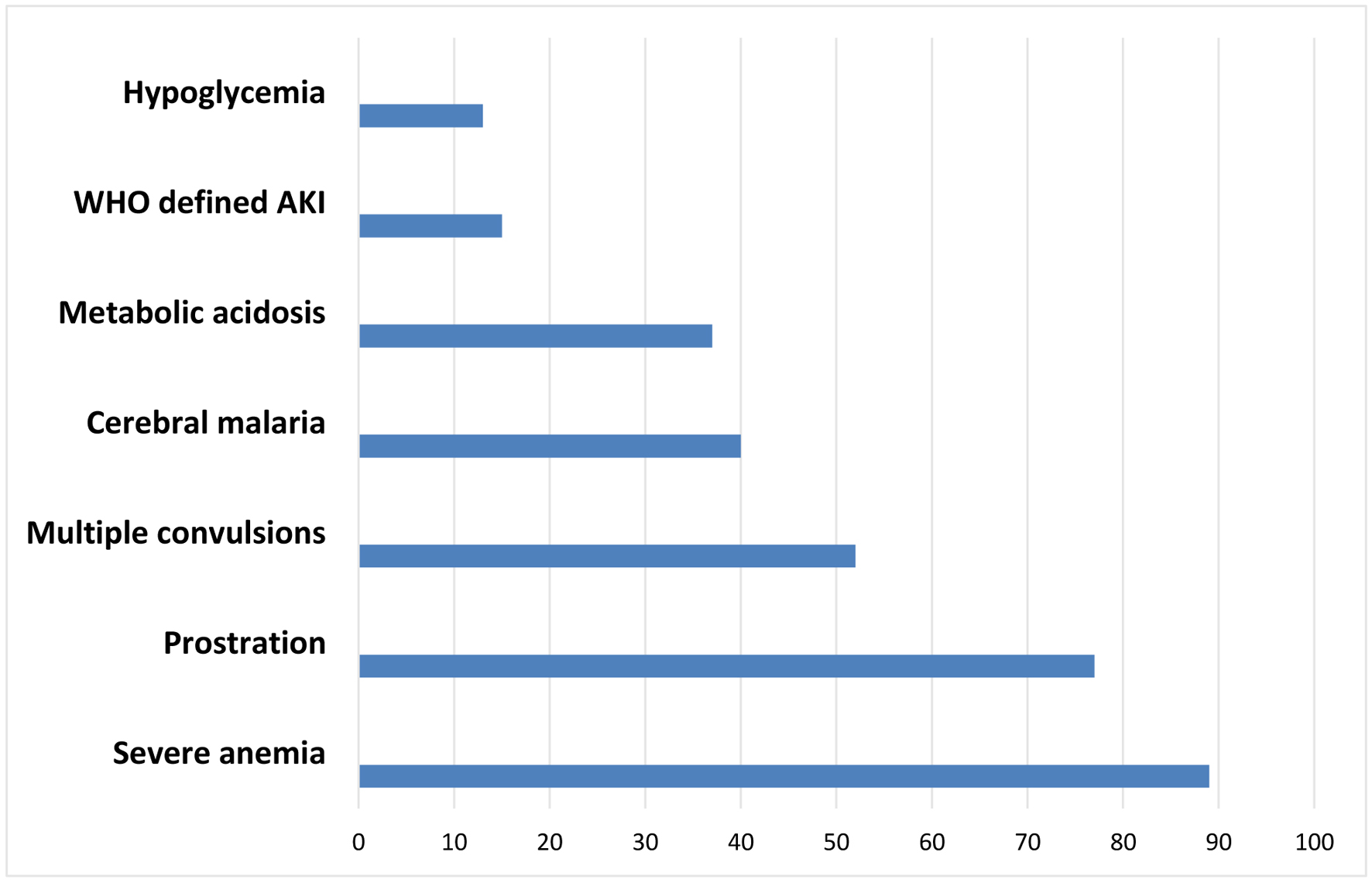 Click for large image | Figure 1. Bar chart showing the features of severe malaria found in the study population. |
Serum cystatin C level at presentation
The overall mean ± SD of serum cystatin C level at admission was 0.74 ± 0.58 mg/L with a range of 0.46 - 3.20 mg/L. The mean serum cystatin C levels for male and female were 0.79 ± 0.69 and 0.68 ± 0.42 mg/L (P = 0.093), respectively as shown in Table 2.
 Click to view | Table 2. Serum Cystatin C Level at Presentation (mg/L) |
Prevalence of AKI in severe malaria using the WHO, KDIGO and cystatin C criteria
The numbers of subjects with AKI diagnosed using Scr-based WHO and KDIGO criteria were 15 and 30, giving a prevalence of 11.9% (95% CI: 8.4 - 24.7) and 23.8% (95% CI: 20.2 - 42.8), respectively. Serum cystatin C-defined AKI was present in 49 subjects, corresponding to a prevalence of 38.9% (P = 0.001; 95% CI: 36.3 - 64.9). This is revealed in Figure 2. The mean ± SD of initial serum creatinine was 1.27 ± 0.80 mg/dL. The number of subjects with AKI diagnosed using initial Scr of > 1.2 mg/dL was 33 (26.2%).
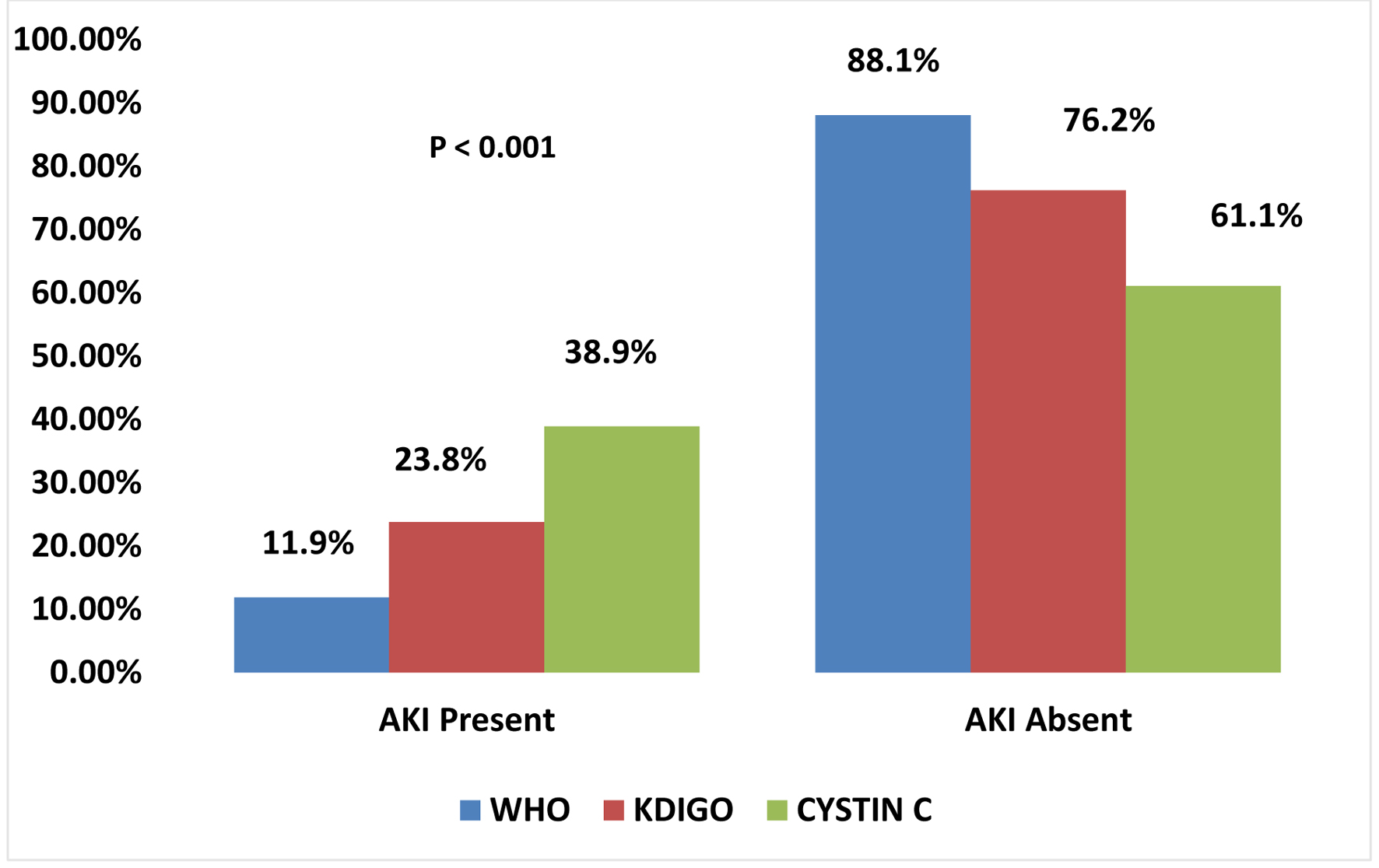 Click for large image | Figure 2. Bar chart showing the prevalence of AKI using WHO, KDIGO and cystatin C. AKI: acute kidney injury; KDIGO: Kidney Disease: Improving Global Outcomes; WHO: World Health Organization. |
Comparison of AKI detection rate of cystatin C and WHO criteria with the KDIGO criteria as the standard
Table 3 shows the AKI detection rate of serum cystatin C compared with the KDIGO and WHO criteria. Of the 30 subjects with KDIGO-defined AKI, 24 of them were also diagnosed by serum cystatin C. The KDIGO criteria were able to detect all the 15 subjects with WHO-defined AKI. Serum cystatin C was able to detect all the 15 subjects that were diagnosed by WHO criteria. There were additional five subjects that developed WHO-defined AKI using the Scr obtained at 36 h who had no WHO-defined AKI at admission. Four (80.0%) of these new five cases had however been earlier detected as AKI by the serum cystatin C which was taken at admission.
 Click to view | Table 3. Comparison of the AKI Detection Rate of Cystatin C and WHO Criteria With the KDIGO Criteria as the Standard |
Sensitivity, specificity, NPV and PPV of cystatin C in the diagnosis of AKI in severe malaria
Model estimation and internal validity test were conducted with the model construction data and serum cystatin C was used to test for validation strength in diagnosing AKI correctly. When serum cystatin C was compared with KDIGO as standard, the sensitivity and specificity were 80.0% and 73.9%, respectively, and the PPV and NPV were 48.9% and 92.2%, respectively. This is further shown in Table 3.
| Discussion | ▴Top |
This study was aimed at determining the predictive value of cystatin C in AKI and evaluating the value of cystatin C in the diagnosis of AKI in severe malaria who were aged 1 - 15 years.
When the prevalences of AKI from the three criteria used in this study were compared, serum cystatin C was able to detect more AKI than both the KDIGO and WHO-defined AKI criteria. This is in keeping with previously reported fact that cystatin C is able to detect subclinical change in renal function better and earlier than creatinine which has several shortfalls in diagnosing AKI [22]. A similar pattern of observation has also been documented by several authors [12, 23]. In addition to being able to detect all subjects with WHO-defined AKI at admission and more than a third of KDIGO in this study, cystatin C (which was done at admission) was also able to detect almost all (80%) those who had no WHO-defined AKI at admission but later developed WHO-defined AKI using the 36-h creatinine. Although the number of subjects in this category was few in this study, a similar observation has been previously documented whereby serum cystatin C detected AKI earlier and better than Scr [23]. A larger longitudinal study of outcome of subjects with cystatin C-defined AKI may be required to further establish this. In this study, KDIGO criteria were able to detect all the subjects who had WHO-defined AKI at admission. This further reiterates the fact that the WHO criteria will only detect severe disease with higher tendency of missing those with the early stage of AKI in severe malaria.
Herbal treatment was the most utilized alternative to orthodox care found in this study. This finding may be ascribed to the rural nature of the study location and the prevailing low socio-economic status of the people as found in this study. The use of herbal treatment may result from factors such as poverty and sociocultural practices [24]. Herbal mixtures have been associated with 35% of all cases of AKI in Africa [25]. They are concocted chemical and biological products which may cause toxic nephropathy by one or combination of several processes depending on the components. These processes include but not limited to direct effect on renal tissues, and hypersensitivity reaction and renal ischemia may also be capable of causing intravascular hemolysis with resultant acute tubular necrosis (ATN) and AKI. Kadiri et al [26] reported traditional herbal remedy as the most common etiology of AKI in adult population, while Anochie et al [27] in a retrospective study also found herbal drugs as second highest factor that contributed to mortality in children with AKI.
The present study found the sensitivity, specificity, NPV and PPV of 80%, 73.9%, 48.9%, and 92.2%, respectively for serum cystatin C compared with AKI defined by KDIGO. These measures of accuracy of serum cystatin C reported in this study could be attributed to the fact that serum cystatin C was compared with KDIGO, a diagnostic standard that utilizes Scr that is froth with several limitations. It is possible that serum cystatin C alone could have done better as a diagnostic tool than when compared with creatinine. Serum cystatin C was able to diagnose 39% out of all the recruited subjects and 80% of subjects diagnosed by the KDIGO. Serum cystatin C assay will therefore diagnose AKI in eight out of every 10 subjects with severe malaria earlier at admission compared with the KDIGO criteria that required a wait period of at least 36 h for the diagnosis to be made. It also has over 90% chance of excluding those that truly do not have AKI.
It is therefore recommended that cystatin C kit should be made available in the institution as part of routine screening test for children with severe malaria for early detection of AKI to capture subjects with subclinical changes in renal function. The limitation of this study was the inability to compare the outcomes of AKI defined by serum cystatin C and creatinine in children with severe malaria.
In conclusion, the sensitivity, specificity and NPV of cystatin C in the early diagnosis of AKI in severe malaria were 80.0%, 73.9% and 92.2%, respectively. Therefore, the serum cystatin C can be used to safely identify children in whom AKI is unlikely, thus leading to shorter duration of admission.
Acknowledgments
The authors acknowledge the contributions of the resident doctors and nursing staff at the CHEW of FMC, Owo, Ondo State, Nigeria for their contributions especially in early identification of potential subjects for recruitment.
Financial Disclosure
There was no financial support of any kind either in grants, equipment or otherwise.
Conflict of Interest
The authors declared that there is no conflict of interest.
Informed Consent
A clear explanation of the study was made to the parent/caregiver and older patients that were conscious following which informed consent from the parents/guardians with assent from children aged 7 years and above were obtained.
Author Contributions
KOO, AOA and ADA conceptualized and designed the title while the other authors were involved in acquisition of data, analysis and interpretation of the data. The article was drafted and revised critically for important intellectual contents by all the authors. Final approval of the version to be published was given by all the authors. The manuscript has been read and approved by all the authors, the requirements for authorship as stated earlier have been met, and each author believes the manuscript represents honest work.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- World Health Organization. World malaria report 2020: 20-years of global report and challenges. Geneva: WHO Press; 2020. Available from: https://www.who.int/publications/i/item/9789240015791 (Accessed March 23, 2023).
- White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383(9918):723-735.
doi pubmed - World Health Organization. The “World malaria report 2022” at a glance. Available from: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (Accessed December 23, 2022).
- World Health Organization. Guidelines for the treatment of malaria, 3rd ed. Geneva: WHO Press; 2015. Available from https://www.afro.who.int/publications/guidelines-treatment-malaria-third-edition (Accessed March 30, 2023).
- Katibi OS, Adedoyin OT, Anoba S, Sowunmi FO, Olorunsola BO, Ibrahim OR. Current trends in the management of acute kidney injury in children. Niger J Paediatr. 2013;40(3):314-320.
- Ramesh M, Mohan B, Murlidharan P, Balan S. Malarial acute kidney injury. Med Sci Int Med J. 2016;6(2):355-356.
- Jallow M, Casals-Pascual C, Ackerman H, Walther B, Walther M, Pinder M, Sisay-Joof F, et al. Clinical features of severe malaria associated with death: a 13-year observational study in the Gambia. PLoS One. 2012;7(9):e45645.
doi pubmed pmc - Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756-766.
doi pubmed - Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138.
- Thanachartwet V, Desakorn V, Sahassananda D, Kyaw Win KK, Supaporn T. Acute renal failure in patients with severe falciparum malaria: using the WHO 2006 and RIFLE criteria. Int J Nephrol. 2013;2013:841518.
doi pubmed pmc - Padhi RK, Mishra S. Incidence of renal involvement in malaria in children of Odisha. ISRN Nephrol. 2013;2013:573735.
doi pubmed pmc - Viswanathan V, Snehalatha C, Nair MB, Ramachandran A. Comparative assessment of cystatin C and creatinine for determining renal function. Indian J Nephrol. 2005;15(3):91-94.
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933-1953.
pubmed - Nigeria and Rockville, USA. NPC and ICF. Available from: https://dhsprogram.com/pubs/pdf/FR359/FR359.pdf (Accessed February 20, 2023).
- Oyinloye MA. Using GIS and remote sensing in urban waste disposal and management: a focus on Owo L.G.A, Ondo State, Nigeria. Eur Int J Sci Technol. 2013;2(7):106-118.
- Olugbamila OB, Omole FK, Omosulu SB, Soyinka OA, Odeyale TO, Olufayo O, Akinrinmade O. Marketing the tourism potentials of Owo community for the development of Ondo State, Nigeria. Res Human Soc Sci. 2012;2(11):83-93.
- Araoye MO. Sample size determination. In: Araoye MO, editor. Research methodology with statistics for health and social sciences. Ilorin: Nathedex Publishers; 2003. p. 115-129.
- Asinobi AO, Ademola AD, Alao MA. Haemodialysis for paediatric acute kidney injury in a low resource setting: experience from a tertiary hospital in South West Nigeria. Clin Kidney J. 2016;9(1):63-68.
doi pubmed pmc - Ogunlesi A, Dedeke I, Kuponiyi O. Socio-economic classification of children attending specialist paediatric centres in Ogun State, Nigeria. Niger Med Pract. 2008;54(1):21-25.
- Afolayan FM. Comparative study on the estimation of glomerular filtration rate using cystatin C and creatinine levels in children with severe malaria and acute kidney injury at the University of Ilorin Teaching Hospital, Ilorin. A Dissertation submitted to National Postgraduate Medical College of Nigeria. May 2017. Available from: http://www.dissertation.npmcn.edu.ng/index.php/FMCPaed/article/view/736.
- Esezobor CI, Iroha E, Oladipo O, Onifade E, Soriyan OO, Akinsulie AO, Temiye EO, et al. Kidney function of HIV-infected children in Lagos, Nigeria: using Filler’s serum cystatin C-based formula. J Int AIDS Soc. 2010;13:17.
doi pubmed pmc - Afolayan FM, Adedoyin OT, Abdulkadir MB, Ibrahim OR, Biliaminu SA, Mokuolu OA, Ojuawo A. Acute kidney injuries in children with severe malaria: a comparative study of diagnostic criteria based on serum cystatin C and creatinine levels. Sultan Qaboos Univ Med J. 2020;20(4):e312-e317.
doi pubmed pmc - Murty MS, Sharma UK, Pandey VB, Kankare SB. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. 2013;23(3):180-183.
doi pubmed pmc - Jha V, Parameswaran S. Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol. 2013;9(5):278-290.
doi pubmed - Liwa AC, Jaka HM. Renal diseases and use of medicinal herbal extracts: a concise update of reported literature in Africa. J Nephrol Renal Ther. 2016;2:008.
doi - Kadiri S, Ogunlesi A, Osinfade K, Akinkugbe OO. The causes and course of acute tubular necrosis in Nigerians. Afr J Med Med Sci. 1992;21(1):91-96.
pubmed - Anochie IC, Eke FU. Acute renal failure in Nigerian children: Port Harcourt experience. Pediatr Nephrol. 2005;20(11):1610-1614.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
