| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Original Article
Volume 2, Number 2, November 2013, pages 47-54
Congenital Nephron Deficit and Chronic Kidney Disease
Mahmoud Kallasha, d, Tyrus Stewartb, Randall Craverc, Vesa Matti Vehaskarib
aPediatric Nephrology Department, Women and Children’s Hospital, University at Buffalo, 219 Bryant Street, Buffalo, NY 14222, USA
bPediatric Nephrology Department, Louisiana State University Health Science Center and Children’s Hospital of New Orleans, 200 Henry Clay Ave, New Orleans, LA 70118, USA
cDepartment of Pathology, Louisiana State University Health Science Center and Children’s Hospital of New Orleans, 200 Henry Clay Ave, New Orleans, LA 70118, USA
dCorresponding author: Mahmoud Kallash, Pediatric Nephrology Department, Women and Children’s Hospital, University at Buffalo, 219 Bryant Street, Buffalo, NY 14222, USA
Manuscript accepted for publication October 18, 2013
Short title: Congenital Nephron Deficit is a Risk Factor for Renal Disease
doi: https://doi.org/10.4021/wjnu112w
| Abstract | ▴Top |
Background: Congenitally low nephron number, often associated with low birth weight, has been shown to be a risk factor for hypertension and cardiovascular disease in humans. Epidemiological studies have also linked it to development of chronic kidney disease (CKD) but this has been difficult to verify experimentally. Previously, we showed that rats with congenitally low nephron number did not develop CKD by 11 months of follow-up. In this study, we tested the hypothesis that animals with congenital nephron deficit have a higher risk for development of kidney disease when exposed to postnatal insults.
Methods: Pregnant Sprague-Dawley rats were exposed to low protein (LP) versus control diet to induce decreased nephron numbers. Offspring from each group were either stressed by unilateral nephrectomy (UN) and high salt diet (HS), or exposed to standard salt diet and sham surgery.
Results: Forty-two percent of stressed animals in both C and LP groups died of renal failure compared to no mortality in non-stressed animals (P = 0.0001). By 6 months of age, non-stressed LP animals did not develop significant renal disease. However, stressed LP animals had significant decrease in kidney function (P = 0.033), high albumin excretion (P = 0.012), and extensive renal histologic damage. The changes tended to be more severe in males.
Conclusions: Congenital low nephron number in our model does not result in CKD by 6 months of life. However, they have increased risk for development of CKD when exposed to postnatal insults, and the risk depends on the severity of deficit.
Keywords: Congenital nephron deficit; High salt diet; Kidney disease; Low birth weight
| Introduction | ▴Top |
Numerous epidemiological studies have shown that adverse prenatal environment and low birth weight are associated with increased risk of hypertension and cardiovascular disease during adulthood [1-3]. This “fetal programming” of adult blood pressure profile has been confirmed by multiple experimental studies in animal models [4-6]. Although several mechanisms have been postulated to be responsible, most studies have focused on the role of total number of nephrons. Even in healthy human populations, there is a wide range in nephron numbers, and the total number has been reported to be low in patients with essential hypertension [7]. In support of a causal role of nephron deficit in the development of hypertension, most experimental models of prenatally programmed hypertension exhibit congenitally low nephron numbers [6, 8, 9].
There is strong epidemiologic evidence that low birth weight, a surrogate for low nephron count, is also a risk factor for development of chronic kidney disease (CKD) in later life, but this has been difficult to demonstrate experimentally. The prevailing hypothesis is that decreased nephron number results in hyperfiltration of the remaining nephrons, causing glomerular sclerosis and systemic hypertension, leading to vicious cycle with further nephron drop-out [10, 11]. It seems logical that if the number of nephrons is low at birth, normal age-related nephron drop-out would increase the risk of later CKD. However, this has not been easy to demonstrate in experimental models, perhaps because there is a large “reserve” pool of nephrons and reaching a critically low number would take an inordinately long observation period. Thus, exposing animals to a postnatal “secondary hit” may accelerate nephron drop-out and unmask the increased risk of CKD.
One might expect the hypertension that develops in several low-nephron animal models to serve as such secondary hit but not all studies have been able to demonstrate this [12]. In a previous study, we exposed rats with congenitally low nephron number to high salt diet, but, despite some evidence of histologic deterioration and being hypertensive, animals did not develop a significant decrease in renal function during an 11-month observation period [4]. Another isolated postnatal insult, uninephrectomy, also failed to result in accelerated CKD in our laboratory (Vehaskari, unpublished observations).
The goal of this study was to determine whether high risk for development of CKD can be unmasked in animals born with low nephron count by the combination of two postnatal insults, one directly reducing the nephron number (uninephrectomy) and the other augmenting hypertension (high salt diet).
| Materials and Methods | ▴Top |
In all experiments, our previously published protocol was followed to induce low birth weight litters [13]. Briefly, pregnant rats in the experimental group were kept on a low protein diet (LP, 6% protein by weight) from gestational day 11 until spontaneous delivery. Dams in the control (C) group remained on a standard 20% protein diet. All litters were reduced to 8 - 11 pups after birth, with equal number of males and females. After weaning on day 21 of life, 50% of C pups and 50% of LP pups were placed on a high salt diet (HS, 3% Na). Because our pilot experiments had shown that long exposure to HS diet was poorly tolerated, these animals were switched to a standard salt diet (SS, 0.3% Na) on day 60 of life. At 4 weeks of age, 50% of the pups in each group underwent uninephrectomy (UN). Thus, the following four experimental groups were created:
1) Maternal control diet + sham operation + SS diet (C-S-SS)
2) Maternal LP diet + sham operation + SS diet (LP-S-SS)
3) Maternal control diet + UN + HS diet days 21-60 (C-UN-HS)
4) Maternal LP diet + UN + HS diet days 21-60 (LP-UN-HS)
At 8 weeks of age, 10 - 12 pups of each group were sacrificed and kidneys were harvested for nitrotyrosine assay. The remaining pups (n = 10-12 in each group) were followed longitudinally.
At 6 months of age, 24-h urine for Cr clearance and urine albumin excretion was performed, and the animals were sacrificed, and both kidneys were harvested for histological semiquantitative assessment of renal injury, following our previous protocol [4]. As a marker of intrarenal oxidative stress, kidney tissue nitrotyrosine content was quantified by immunoblotting following our previously established protocol [13]. The total number of glomeruli per kidney was determined at the age of 5 - 6 weeks by the maceration method as in our pervious protocol [14]. Urine Cr and albumin concentration was measured by a standard ELISA kit, while plasma creatinine concentration was measured by a standard autoanalyzer.
Statistical analysis
Statistical comparisons were performed by analysis of variance (ANOVA) with Dunnett’s post-test or Fisher’s exact test whenever appropriate, comparing each group to C-S-SS. P value of < 0.05 is considered statistically significant.
| Results | ▴Top |
Birth weight and number of glomeruli
LP litters had a 26 % lower birth weight compared to C pups (mean 5.69 g vs. 7.72 g, respectively, P = 0.0001; Fig. 1). The mean number of glomeruli per kidney was 21,420 in LP group vs. 26,630 in C group (20% reduction, P = 0.002; Fig. 2).
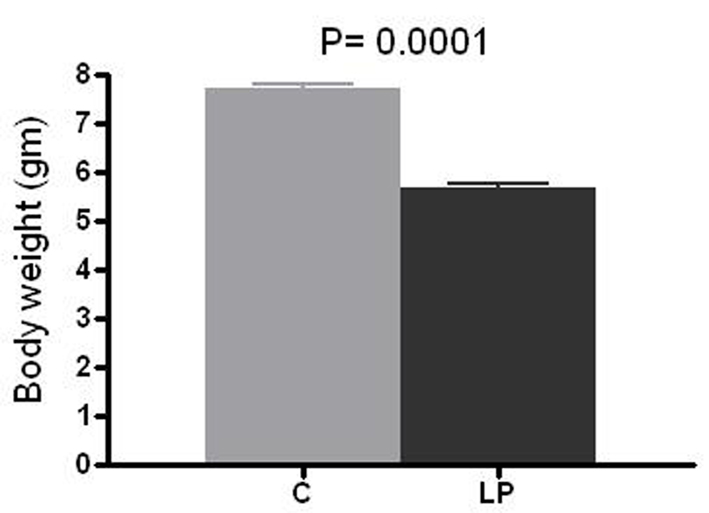 Click for large image | Figure 1. Birth weight. Body weight was measured at day 1-2 of life in prenatally control (C) and low protein (LP) diet animals. Data presented as mean ± SEM (n = 11-12 in each group). |
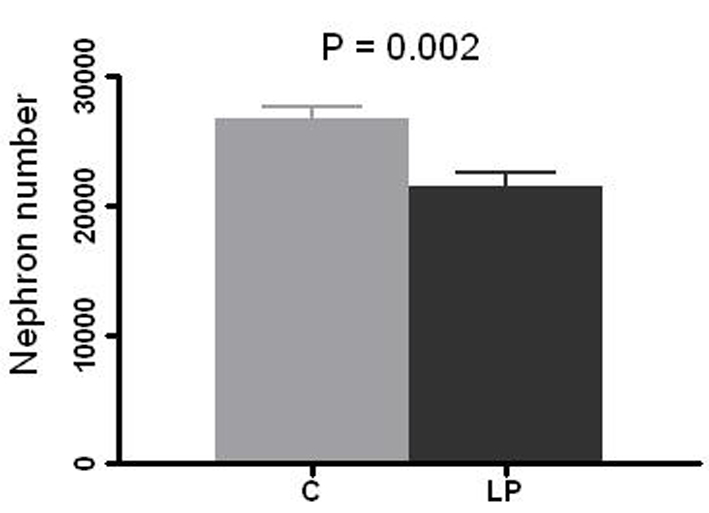 Click for large image | Figure 2. Nephron number per kidney. Right kidney in prenatally control (C) and low protein (LP) diet rats was resected at 4 - 5 weeks of age and glomeruli count was performed by the maceration method. Data presented as mean ± SEM (n = 10 in each group). |
Animal survival
There were no deaths in C-S-SS or LP-S-SS group by 6 months of age. In contrast, 5 of 11 animals in C-UN-HS and 5 of 12 animals in LP-UN-HS died; there was no significant difference between these two groups. Except for one female in the LP-UN-HS group, all deaths occurred in male rats. Before death, these animals showed signs of uremia such as progressive edema and decreased food consumption. Serial plasma samples were available from three animals and kidney failure as the cause of death was confirmed by progressive rise in plasma creatinine (peak 7.8 - 8.5 mg/dL) in all three animals (Fig. 3).
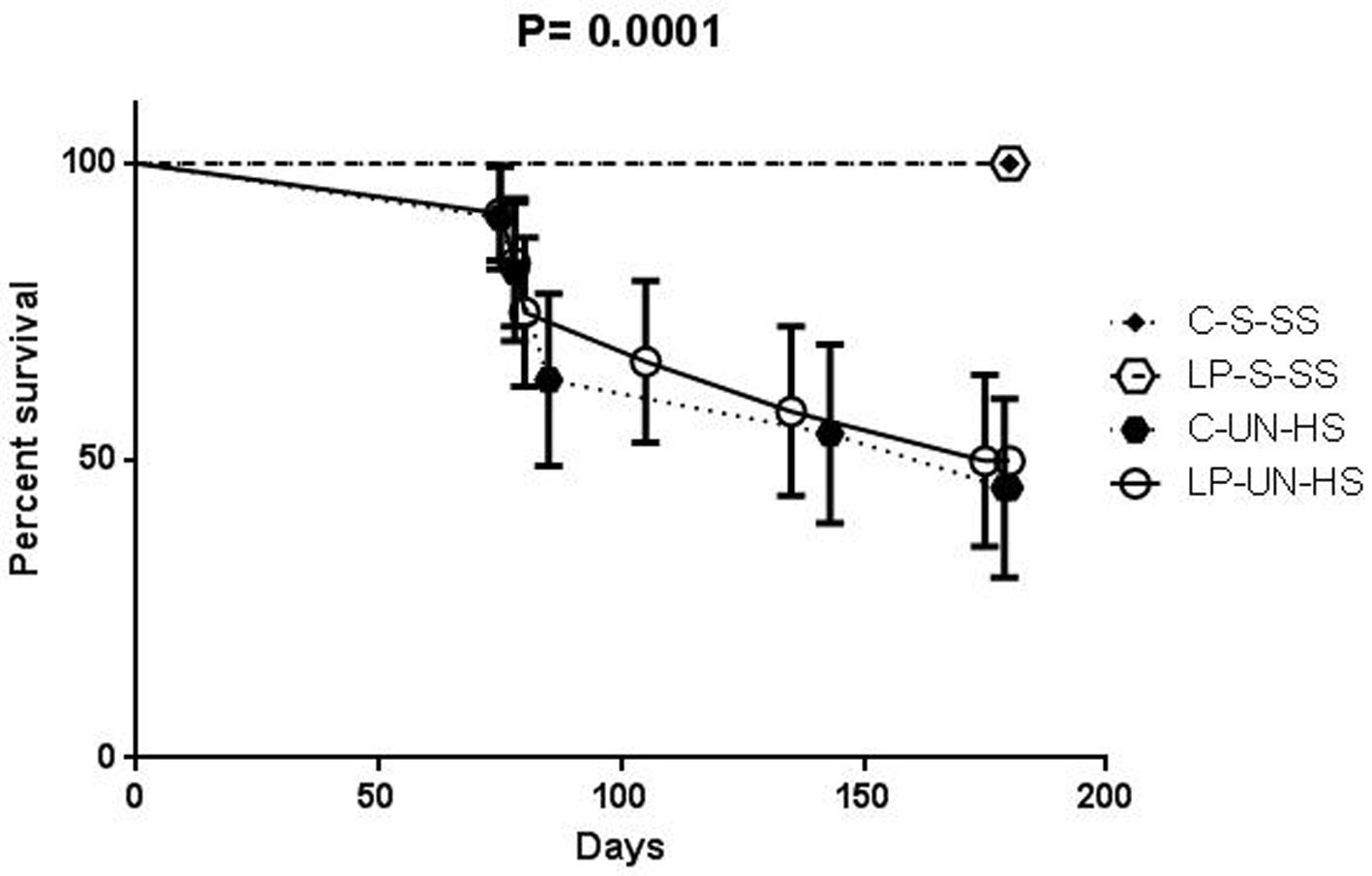 Click for large image | Figure 3. Survival curve. Survival in animals of the four groups over the 6 months of follow-up. C: prenatal control diet; LP: prenatal low protein diet; S: sham surgery; UN: uninephrectomy; SS: standard salt diet postnatally; HS: high salt diet on day 21-60 of life (n = 11-12 in each group, analysis was performed by Fisher’s test). |
Blood pressure
Blood pressures were examined at 2 and 6 months of age by a tail cuff method. At 2 months, while both HS groups were still on their designated diets, they were hypertensive (P < 0.0001), and the degree of hypertension was similar in C and LP animals despite the difference in nephron numbers (Fig. 4a). Because only four males in the two HS groups survived to 6 months, the 6-month comparison was done in females only. As illustrated in Figure 4b, female rats in both UN-HS groups were hypertensive (17 - 20 mmHg higher than C-S-SS animals) despite the fact that the HS diet had been discontinued 26 weeks earlier (P < 0.014). Again, there was no significant difference between LP-UN-HS and C-UN-HS (mean BP 138 ± 17 vs. 134 ± 10 mmHg, respectively). Thus, UN combined with HS appeared to be critical for development and maintenance of hypertension, rather than prenatally low nephron number.
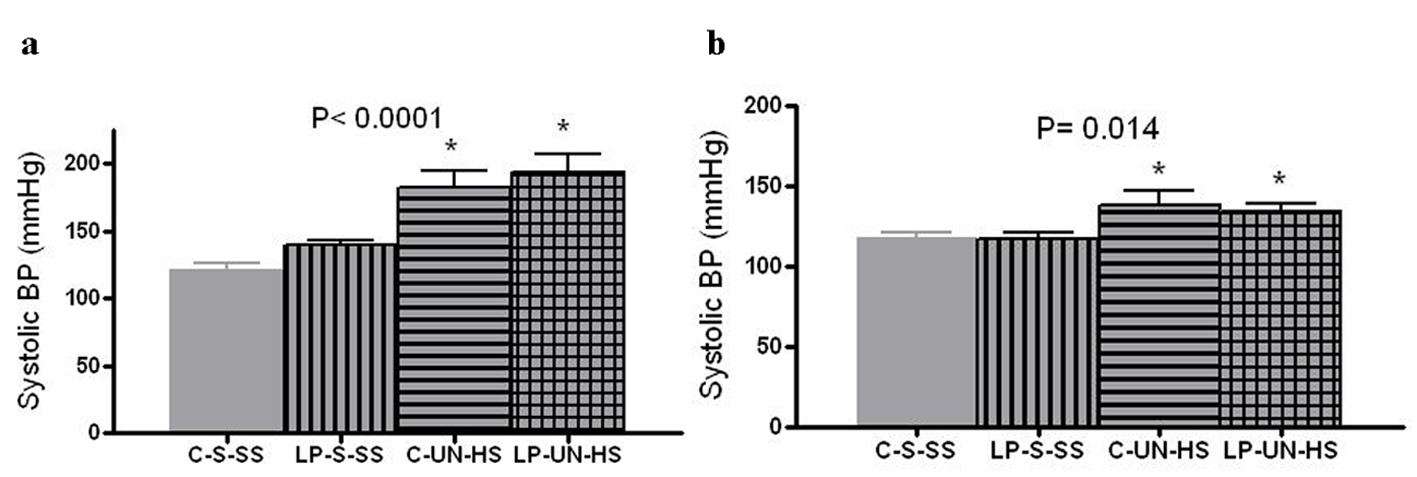 Click for large image | Figure 4. Systolic blood pressure. Tail cuff blood pressure at (a) 2 months (n = 10-12 in each group) and in (b) 6 months old females (n = 5-7 in each group). C: prenatal control diet; LP: prenatal low protein diet; S: sham surgery; UN: uninephrectomy; SS: standard salt diet postnatally; HS: high salt diet on day 21-60 of life (*P < 0.05 vs. C-S-SS). |
Renal function
Creatinine clearance results were normalized to 100 g of body weight. At 6 months, creatinine clearance in females was highest in the C-S-SS group (0.6 mL/min/100 g) and lowest in the LP-UN-HS group (0.28 mL/min/100 g at 47% of the control group), with C-UN-HS and LP-S-SS animals exhibiting intermediate values (63% of C-S-SS) (Fig. 5, P = 0.033). To estimate the frequency of renal functional impairment in both sexes, CKD was defined as CrCl of < 50% of the control group (C-S-SS) or as death before 6 months of age. Defined this manner, CKD developed in 0/12 in C-S-SS, 2/12 animals in LP-S-SS, 7/11 animals in C-UN-HS, and 9/12 animals in LP-UN-HS group (P = 0.01).
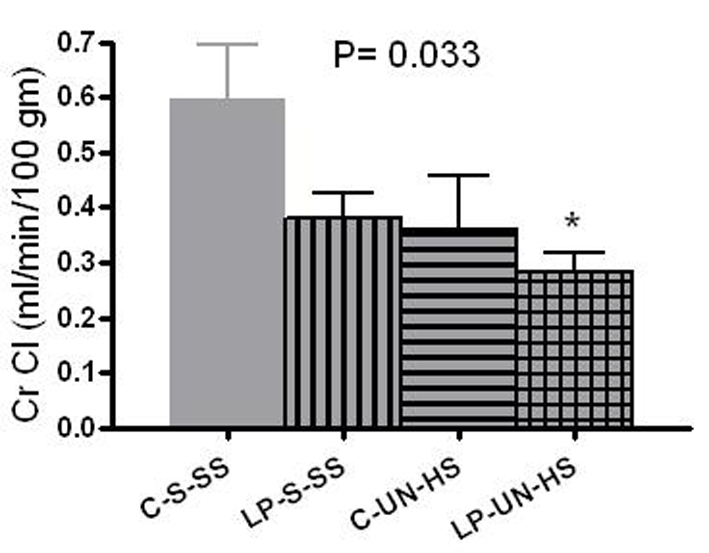 Click for large image | Figure 5. Renal function. Renal function was measured by creatinine clearance (CrCl) in 6 month old females (mL/min/100 g of body weight). C: prenatal control diet; LP: prenatal low protein diet; S: sham surgery; UN: uninephrectomy; SS: standard salt diet postnatally; HS: high salt diet on day 21-60 of life (n = 5-7 in each group,*P < 0.05 vs. C-S-SS). |
Albuminuria
Albuminuria is presented as albumin/creatinine ratio (mg/mg). At 6 months of age, analysis was limited to the surviving females only. Significant differences were evident, from the lowest in C-S-SS (0.8 ± 0.55 mg/mg) to the highest in LP-UN-HS (2.4 ± 0.9 mg/mg) (Fig. 6, P = 0.012).
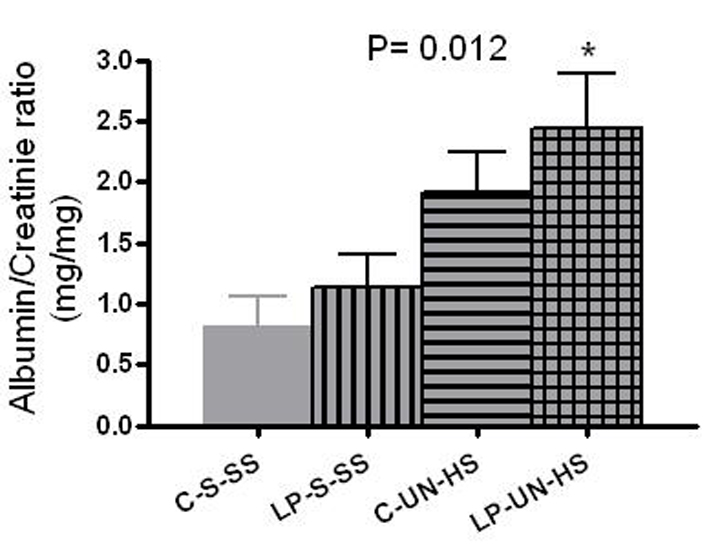 Click for large image | Figure 6. Albumin excresion. Albumin excretion in 6 months old females, measured as albumin/creatinine ratio (mg/mg). C: prenatal control diet; LP: prenatal low protein diet; S: sham surgery; UN: uninephrectomy; SS: standard salt diet postnatally; HS: high salt diet on day 21-60 of life (n = 5-7 in each group, *P < 0.05 vs. C-S-SS). |
Pathology
Kidney morphology was examined at 6 months or at death for animals that died before 6 months. LP-S-SS animals had significantly lower kidney/body weight compared to C-S-SS animals (0.296 ± 0.03 vs. 0.655 ± 0.033 mg/g respectively, P = 0.017). As expected, animals exposed to UN had compensatory hypertrophy of the remaining kidney (0.6 ± 0.05 mg/g in LP-UN-HS vs. 0.81 ± 0.37 mg/g in C-UN-HS), and there was no significant difference between the two UN groups. The results of semiqualitative determination of histologic injury are presented in Table 1. For each of the scored items, the two SS groups had the least injury whereas the two HS groups showed the most injury. Although the injury scores in LP-UN-HS were numerically higher than those in C-UN-HS, the difference was not statistically significant. For both glomerular and tubulointerstitial injury scores, males had generally higher injury scores but it did not reach statistical difference (Fig. 7).
 Click to view | Table 1. Kidney Pathology at 6 Months of Age |
 Click for large image | Figure 7. Kidney pathology at 6 months of age. Combined glomerular injury (a) and tubulointerstitial injury score (b) in control (C) and LP rats after exposure to high salt diet (HS) and unilateral nephrectomy (UN) vs. sham (S) surgery and on standard salt diet. (n = 6-9 in each group, *P < 0.05 vs. C-S-SS). |
Oxidative stress
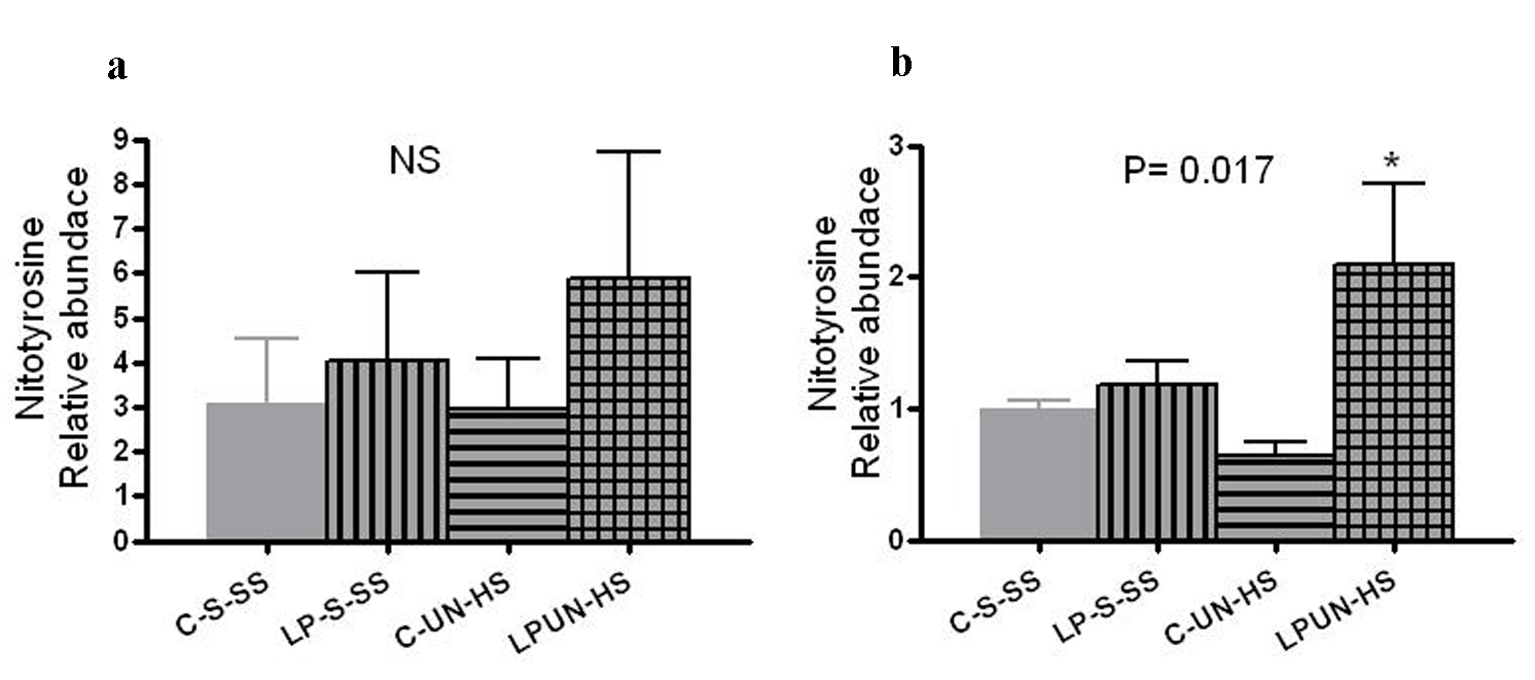
Tissue oxidative stress level can be estimated by the amount of nitrosylated tyrosine residues on tissue proteins, reflecting the activity of reactive oxygen species. At 8 weeks, LP animals had higher abundance of renal nitrotyrosine content although it did not reach statistical difference (Fig. 8a). However, at 6 months of age, there was significant increase in the LP-UN-HS female animals (Fig. 8b, P = 0.017).
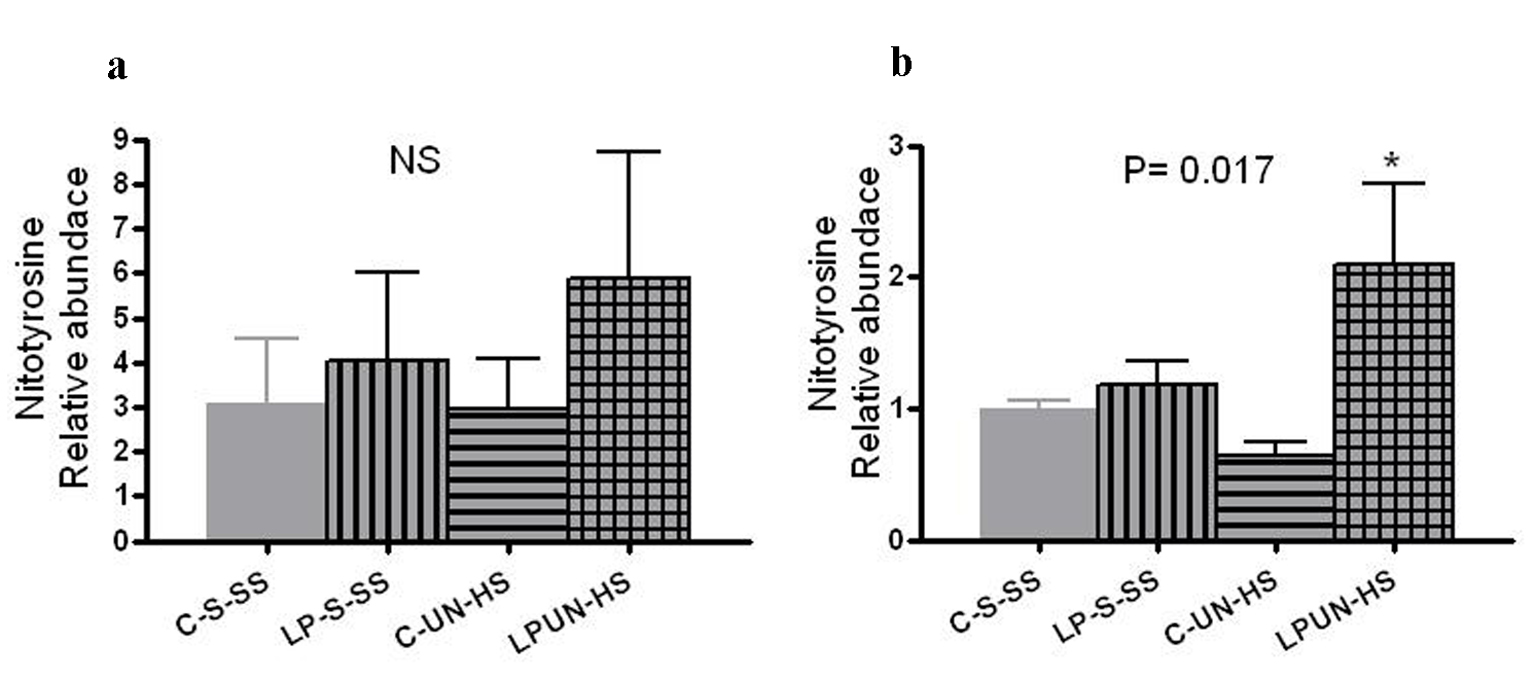 Click for large image | Figure 8. Renal nitrotyrosine content. Kidneys from (a) 8 weeks old offspring (n = 8 in each group) and in (b) 6 months old females (n = 5-7 in each group, *P < 0.05 vs. C-S-SS). |
| Discussion | ▴Top |
This study was based on the hypothesis that exposing animals with congenitally low nephron numbers to postnatal renal injuries results in accelerated development of CKD. The results clearly show that LP animals exposed to the combination of postnatal uninephrectomy and high salt diet leads to early adult life CKD which tends to be more severe in males. Without the postnatal insults, LP animals exhibited only minor signs of histologic renal damage and non-significantly reduced renal function by 6 months of age. Unexpectedly, however, exposure of control animals to the same postnatal insults resulted in a relatively severe renal damage, although it seemed to be less severe than the renal damage in LP-UN-HS animals.
The conventional theory linking congenitally low nephron count to CKD proposes that the remaining fewer nephrons compensate by hyperfiltering which is ultimately detrimental, causing glomerulosclerosis and progressive nephron dropout [11, 15, 16]. However, the outcome in our LP-S-SS group showed that approximately 20% prenatal reduction in total nephron count did not lead to early decline in renal function without additional postnatal adverse factors. Indeed, to demonstrate that pure hyperfiltration injury causes kidney failure, a 75% reduction in number of nephrons in the rat may be required [17]. Therefore, lesser degrees of nephron deficit may serve as a risk factor but may need additional injurious events to become clinically significant.
Hypertension commonly accompanies CKD and contributes to the progression of the disease. Most earlier studies on rats with low birth weight and congenitally low nephron number reported elevated blood pressures, measured by tail cuff plethysmography [5, 18-21]. However, the presence of sustained hypertension in models such as ours remains controversial. Some studies, employing tail cuff methodology [12] and more recently 24-h blood pressure monitoring by intra-arterial telemetry [22, 23], have been unable to confirm the presence of hypertension. Besides methodological differences, the contradicting results may reflect differences in response to the stress induced by the measurements [19, 24]. In the present study, we observed no differences between control and LP animals with or without salt loading but, interestingly, early life HS seemed to induce life-long hypertension in all UN rats independently of nephron number at birth. Our results suggest that the combined impact of postnatal UN and HS far exceeded that of congenital reduction of nephrons.
Because hypertension may not be a critical factor in the development of CKD in our model, other possible detrimental effects of salt should be considered. High Na environment may have a direct vasoconstrictive effect on systemic vasculature and hence contribute to tissue injury [25-27], and could increase renal oxidative stress [4, 28, 29]. In the present study, the highest renal tissue oxidative stress levels were seen in the LP rats, but it only reached statistical significance in the LP-UN-HS group at 6 months of age. This suggests that animals with congenitally low nephron number are intrinsically more prone to excessive oxidative stress, consistent with results of other researchers [30, 31]. However, it does not appear to be a major factor in the development of CKD under the conditions of this study.
Although our study was not designed to evaluate sex differences in development of CKD, males developed more severe renal disease than females. Mortality was significantly higher in males, and they tended to have more severe albuminuria, renal oxidative stress, and advanced glomerular and tubulointerstitial sclerosis compared to females. Other investigators have reported similar sex differences in development of hypertension and kidney disease [32-34], and in renal oxidative stress [31].
In conclusion, our results indicate that rats with congenitally mild nephron deficit (20% reduction) do not develop impaired renal function during the first 6 months of life, despite the presence of hyperfiltration. However, when additional postnatal exposures were imposed, CKD ensued and was worse in males. Since humans exhibit a wide range of nephron number, those at the low end of the spectrum have a higher risk for CKD when exposed to environmental insults.
Animal Care
The study protocol was approved by the Institutional Animal Care and Use Committee of the Research Institute for Children, and all experiments were conducted in accordance with the NIH guide to the care and use of laboratory animals. Animals used: Sprague-Dawley rats for all experiments.
Financial Support
This study was awarded the grant from the National Kidney Foundation, Louisiana Chapter, 2011. Award amount: USD 10,000.
Conflict of Interest
The authors have declared that no conflict of interest exists. All authors certify that he or she has participated sufficiently in the intellectual content, the analysis of data. Each author has reviewed the final version of the manuscript and approves it for publication.
| References | ▴Top |
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564-567.
doi pubmed - Yiu V, Buka S, Zurakowski D, McCormick M, Brenner B, Jabs K. Relationship between birthweight and blood pressure in childhood. Am J Kidney Dis. 1999;33(2):253-260.
doi - Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci. 2010;17(2):186-195.
doi pubmed - Stewart T, Ascani J, Craver RD, Vehaskari VM. Role of postnatal dietary sodium in prenatally programmed hypertension. Pediatr Nephrol. 2009;24(9):1727-1733.
doi pubmed - Habib S, Gattineni J, Twombley K, Baum M. Evidence that prenatal programming of hypertension by dietary protein deprivation is mediated by fetal glucocorticoid exposure. Am J Hypertens. 2011;24(1):96-101.
doi pubmed - Vehaskari VM. Programming of hypertension: the nervous kidney. Am J Physiol Renal Physiol. 2008;295(1):F27-28.
doi pubmed - Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348(2):101-108.
doi pubmed - Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59(5):1663-1669.
doi pubmed - Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008;74(2):187-195.
doi pubmed - Ritz E, Amann K, Koleganova N, Benz K. Prenatal programming-effects on blood pressure and renal function. Nat Rev Nephrol. 2011;7(3):137-144.
doi pubmed - Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549(Pt 3):929-935.
doi pubmed - Zimanyi MA, Bertram JF, Black MJ. Does a nephron deficit in rats predispose to salt-sensitive hypertension? Kidney Blood Press Res. 2004;27(4):239-247.
doi pubmed - Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68(5):2180-2188.
doi pubmed - Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59(1):238-245.
doi pubmed - Azuma H, Nadeau K, Mackenzie HS, Brenner BM, Tilney NL. Nephron mass modulates the hemodynamic, cellular, and molecular response of the rat renal allograft. Transplantation. 1997;63(4):519-528.
doi pubmed - Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol. 2001;12(6):1315-1325.
pubmed - Suzuki H, Tokuriki T, Saito K, Hishida A, Suzuki K. Glomerular hyperfiltration and hypertrophy in the rat hypoplastic kidney as a model of oligomeganephronic disease. Nephrol Dial Transplant. 2005;20(7):1362-1369.
doi pubmed - Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol. 2001;16(5):417-422.
doi - Augustyniak RA, Singh K, Zeldes D, Singh M, Rossi NF. Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1375-1382.
doi pubmed - Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64(11):965-974.
doi - Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65(4):1339-1348.
doi pubmed - Holemans K, Gerber R, Meurrens K, De Clerck F, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr. 1999;81(1):73-79.
pubmed - Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R453-461.
doi pubmed - O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. J Endocrinol. 2008;196(2):343-352.
doi pubmed - Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, et al. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol. 2008;294(3):H1258-1265.
doi pubmed - Payne JA, Alexander BT, Khalil RA. Decreased endothelium-dependent NO-cGMP vascular relaxation and hypertension in growth-restricted rats on a high-salt diet. Hypertension. 2004;43(2):420-427.
doi pubmed - Giardina JB, Green GM, Rinewalt AN, Granger JP, Khalil RA. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension. 2001;37(2 Pt 2):516-523.
doi pubmed - Taylor NE, Glocka P, Liang M, Cowley AW, Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47(4):692-698.
doi pubmed - Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, et al. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012;15(2):201-208.
doi pubmed - Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Le NL, Pladys P, et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1236-1245.
doi pubmed - Ojeda NB. Low birth weight increases susceptibility to renal injury in a rat model of mild ischemia-reperfusion. Am J Physiol Renal Physiol. 2011;301(2):F420-426.
doi pubmed - Wesseling S, Koeners MP, Joles JA. Salt sensitivity of blood pressure: developmental and sex-related effects. Am J Clin Nutr. 2011;94(6 Suppl):1928S-1932S.
doi pubmed - Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530(Pt 1):141-152.
doi pubmed - Thone-Reineke C, Kalk P, Dorn M, Klaus S, Simon K, Pfab T, Godes M, et al. High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1025-1030.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
