| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Original Article
Volume 1, Number 2-3, June 2012, pages 66-72
Association of Hyperuricemia With Carotid Intima-Media Thickness, Albuminuria, Diabetes, Hypertension in Chronic Renal Failure
Jayanta Paula, b, Somnath Dasguptaa
aDepartment of Medicine, Burdwan Medical College, West Bengal, India
bCorresponding author: Dr Jayanta Paul, J+B Lodge, Sontosh Sarani, Banamali Pur, Barasat, North 24 Parganas, West Bengal, PIN: 700124, India
Manuscript accepted for publication June 12, 2012
Short title: Association of Hyperuricemia
doi: https://doi.org/10.4021/wjnu14w
| Abstract | ▴Top |
Background: Carotid artery intima-media thickness (CAIMT) measured by B-mode ultrasonography is widely used as a surrogate marker atherosclerosis. Hyperuricemia is also a well recognized risk factor for cardiovascular diseases. This study was done to find out the role of hyperuricemia on CAIMT and albuminuria, diabetes and hypertension in chronic renal failure (CRF) patients.
Methods: 132 CRF patients and 66 age and sex matched healthy controls were included in this study. Out of 132 CRF patients, 35 were hyperuricemic. CAIMT were measured by B-mode ultrasonography. Statistical analyses were done by SPSS (Statistical package for the social Sciences) soft ware (window version 17.0).
Results: Hyperuricemia was independently correlated with CAIMT in CRF patients (P = 0.018). CRF patients with hyperuricemia had significantly higher CAIMT compared to CAIMT of CRF patients without hyperuricemia (P value < 0.001) and healthy controls (P < 0.001). In hyperuricemic CRF patients, prevalence of hypertension (P < 0.001) and diabetes (P = 0.007) was significantly higher than non-hyperuricemic CRF patients.
Conclusions: Hyperuricemia is independently responsible for increased thickening of Carotid artery intima media thickness and also associated with higher level of albuminuria, and higher prevalence of hypertension and diabetes in CRF patients.
Keywords: Carotid artery intima media thickness; Chronic renal failure; Hyperuricemia; Diabetes; Hypertension; Albuminuria
| Introduction | ▴Top |
Increased thickening of carotid artery intima media thickness (CAIMT) is a mirror of generalized atherosclerosis [1]. CAIMT measured noninvasively by ultrasonography is widely used as a surrogate marker of atherosclerosis [2]. Hyperuricemia (HUA) is an independent risk factor for the progression of renal dysfunction [3, 4] and cardiovascular events [3-5], and 18.4% of female and 25.8% of male patients with hyperuricemia is suffering from chronic renal failure (CRF) [6]. The National Health Examination Survey III-USA demonstrated that serum uric acid (SUA) level > 5.7 mg/dL is an independent risk factor for Coronary artery disease (CAD) and > 7 mg/dL is an independent risk factor for stroke [7]. A higher SUA level is an atherogenic factor [2] but some study did not include the effect of hyperuricemia on CAIMT [8]. Several studies have shown the association between hyperuricemia and microalbuminuria [9] but some study showed there was lack of relationship between SUA and proteinuria [10].
This study was done to find out the role of hyperuricemia on CAIMT and albuminuria in CRF patients.
| Materials and Methods | ▴Top |
Study subjects
The study subjects included 132 CRF patients, and 62 age and sex matched healthy controls.
CRF patients
Out of 132 CRF patients, 35 were suffering from hyperuricemia, 47 (35.6%) of CRF patients were female and 85 (64.4%) were male. Mean age of CRF patients was 45.96 ± 0.92 years, 59 (44.7%) were smokers and 73 (55.3%) were non-smokers, 36 (27.3%) and 35 (26.5%) were suffering from dyslipidemia and hyperuricemia respectively, 37 (28%) glomerular filtration rate (GFR) patients had Diabetes. Mean 24 hours total urine albumin excretion and GRF were 784.43 ± 45.23 mg and 17.47 ± 0.52 mL/min/1.73 m2 respectively, 80 (60.6%) were suffering from hypertension (Table 1, 2).
 Click to view | Table 1. Demographic Characters and Clinical Data of CRF Patients and Healthy Controls |
 Click to view | Table 2. Demographic Characters and Clinical Data of Hyperuricemic and Non-Hyperuricemic CRF Patients |
Healthy controls
Out of 62 healthy controls, 39 (59.1%) male and remaining were female. Mean age of the healthy controls was 46.74 ± 1.19 years. All healthy controls were non smokers, non-dyslipidemic, non-hyperuricemic and non-diabetic non-hypertensive, 24 hours total urine albumin excretion was not measurable (Table 1).
After taking consent, all subjects underwent a careful interview, a clinical examination with an evaluation of patient history based on hospital and outpatients records and laboratory investigations.
Both CRF patients and healthy controls had normal body mass index, body weight and waist circumference.
Biochemical measurements
Venous blood was taken in the morning after an overnight fast for at least 12 hours for biochemical analysis. All biochemical tests were performed at department of biochemistry, Burdwan Medical College.
Uric acid measurement
Hyperuricemia was diagnosed when serum uric acid level was > 7 mg/dL in men and > 6.5 mg/dL in women [11]. Uric acid was measured on a standard auto analyzer.
GFR measurement
CRF was identified when glomerular filtration rate (GFR) was < 60 mL/min/1.73 m2 for consecutive 3 months or more [10]. Glomerular filtration rate (GFR) was estimated with The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula which includes serum creatinine, age, gender and race as variables, with different versions depending on ethnicity, gender and creatinine value [12].
Twenty four (24) hours urine total albumin excretion measurement
Twenty four (24) hours urine total albumin excretion is the “gold stander “for measurement of albuminuria [13] and was measured by Esbach’s albuninometer. Microalbuminuria is defined when 24 hours total urine albumin excretion is 30 - 300 mg and when it is > 300 mg, it is called proteinuria or macroalbuminuria [14].
Plasma glucose measurement
Plasma glucose was estimated by a “glucose oxidase-peroxidase” method. Diabetes was diagnosed according to “American Diabetes Association” when a previous or current 12 h fasting glucose level is 7 mmol/L or greater (≥ 126 mg %).
Lipid profile
Serum total cholesterol was measured by “cholesterol oxidase-peroxidase” method. Patients who used cholesterol lowering medication or had a total serum cholesterol level ≥ 200 mg/dL [15] or triglycerides > 200 mg/dL [16] or HDL-C < 40 [17] were classified as having Dyslipidemia.
Anthropometric measurements
Body mass index measurement
Body mass index (BMI) was calculated by using the formula: BMI = weight (in kilograms)/height (in meters)2. All CRF patients and healthy controls were non-obese (Obesity was defined as a body mass index (BMI) of ≥ 25 kg/m2) [17].
Blood pressure estimation
Hypertension was diagnosed when a patient had systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg after taking 5 minutes rest or had received medicine for hypertension [18]. The average of three BP measurements was used for final estimation of BP.
CAIMT measurement
CAIMT was performed with a 7 MHz B-mode ultrasound system (Philips-HD7 Diagnostic Ultrasound System, made in China). The IMT was defined as the distance between the leading edge of the luminal echo to the leading edge of the adventitia of the media, was measured at the diastolic phase (Fig. 1). The B-mode scanning protocol included the scanning of the right and left common carotid arteries (3 cm before the carotid bifurcation), carotid bifurcation, as well as of the internal carotid artery 2 cm distally from the carotid bifurcation [19]. Measurements were always performed in plaque-free arterial segments [20].
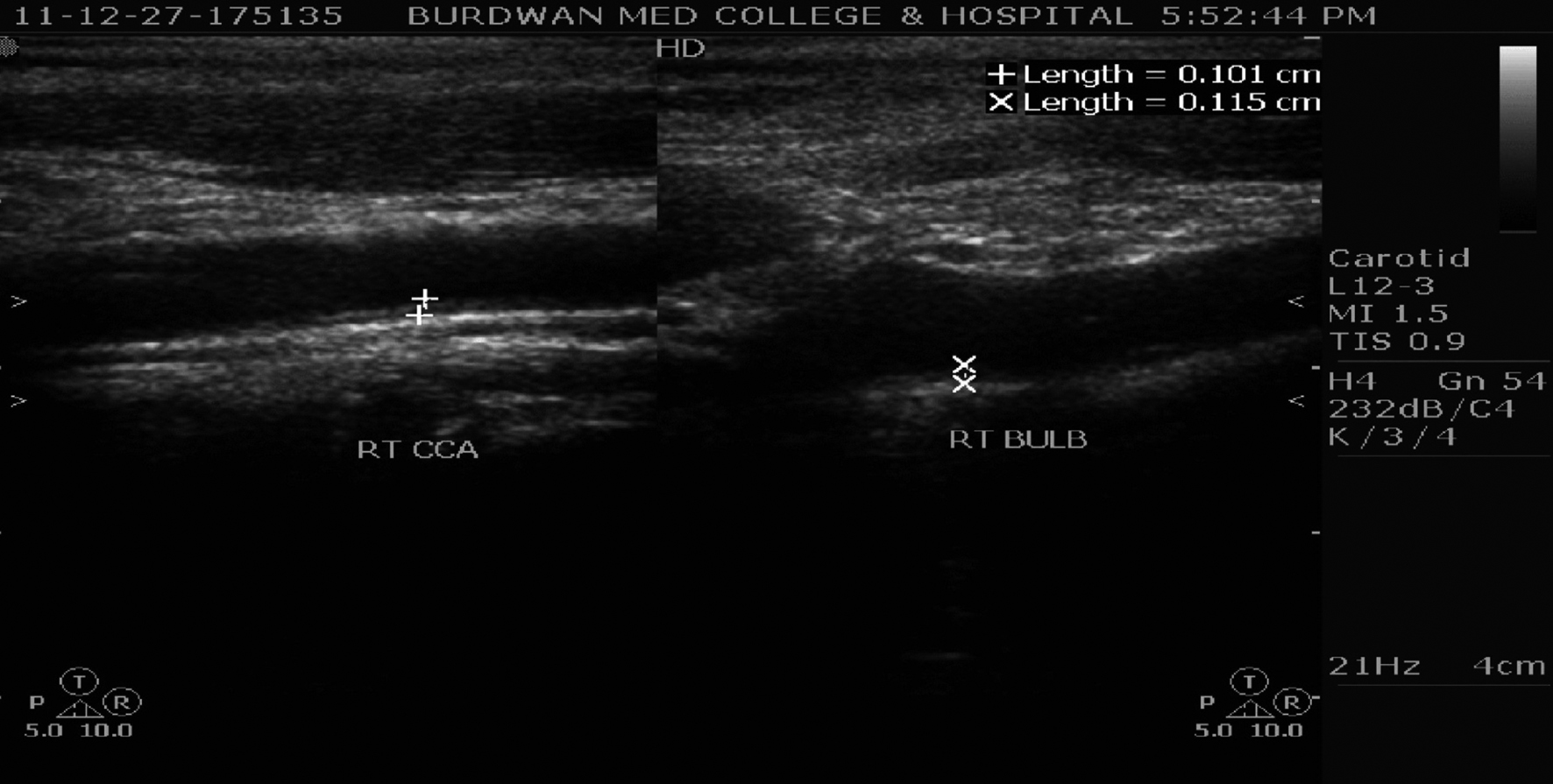 Click for large image | Figure 1. Carotid artery intima media thickness at right common carotid artery (RT CCA) and right carotid bifurcation (RT BULB) in a hyperuricemic CRF patient. |
Others
Hemodialysis
Hemodialyzed patients were identified as who had been on regular hemodialysis treatment (treated thrice weekly) for at least 6 months [21].
Smoking
Participants were classified as non-smokers if they responded that they had smoked fewer than 100 cigarettes or 5 packs of cigarettes during their lifetime [22].
All examinations and measurements were performed by same examiner to exclude examiner bias.
Statistical methods
Chi-square test, Paired t test, independent samples t test, linear multivariate regression analysis with 95 percent confidence interval were performed for data analysis. P value less than 0.05 was taken as statistically significant. Data were expressed as means ± SE (standard Error). All these analysis were performed using a commercially available software SPSS (17th version for window) on personal computer.
| Results | ▴Top |
Here CRF patients and healthy controls were age (P value = 0.605) and sex (P value = 0.467) matched. CRF patients with hyperuricemia and without hyperuricemia were also age (P value = 0.380) and sex (P value = 0.074) matched.
There was no difference of GFR between CRF patients with hyperuricemia and without hyperuricemia (P value = 0.603).
In this study, 45.71% hyperuricemic CRF patients had diabetes and mean fasting blood sugar in this group 134.88 ± 7.42 mg/dl. 85.71% and 57.14 % CRF patients with hyperuricemia were associated with hypertension and dyslipidemia respectively.
In our study, CRF patients with hyperuricemia had significantly higher CAIMT (1051.22 ± 15.60 micron, P value = 0.000) compared to CAIMT of CRF patients without hyperuricemia (857.56 ± 15.95 micron) (Table 3).
 Click to view | Table 3. Comparison of CAIMT of Different Groups by Independent Samples t Test |
It was also shown that hyperuricemic CRF patients had higher CAIMT (1051.22 ± 15.60 micron, P value = 0.000) than age (P value = 0.786) and sex (P value = 0.058) matched healthy controls (707.16 ± 6.79 micron) (Table 3).
In CRF patients, multivariate regression analysis showed CAIMT was positively correlated with age (P = 0.044), sex (P = 0.07), smoking (P = 0.004), dyslipidemia (P = 0.001), hypertension (P = 0.002), dialysis (P = 0.004), 24 hours total urine albumin excretion (P = 0.002), fasting blood sugar (P = 0.000) and hyperuricemia (P = 0.018), and negatively correlated with GRF (P = 0.000). So, hyperuricemia was independently correlated with CAIMT in CRF patients (Table 4).
 Click to view | Table 4. Linear Multivariate Regression Analysis of Traditional Risk Factors Correlating With CAIMT in CRF Patients. |
Our study documented that hyperuricemic CRF patients had higher albuminuria (1148.71 ± 81.19 g/24 hours, P value = 0.000) compared to CRF patients without hyperuricemia (652.99 ± 47.73 mg/24 hours).
In hyperuricemic CRF patients, prevalence of hypertension was significantly higher (85.71% P value = 0.000) than non-hyperuricemic CRF patients (51.54%).
Hyperuricemic CRF patients had significantly higher fasting blood sugar (134.88 ± 7.42 mg/dL, P value = 0.000) than CRF patients without hyperuricemia (102.40 ± 2.89 mg/dL). There were also higher prevalence of fasting blood sugar in CRF patients with hyperuricemia than CRF patients without hyperuricemia (P value = 0.007).
| Discussion | ▴Top |
Kawamato et al [23] and Neogi T et al [24] found that SUA is an independent risk factor of atherosclerosis along with known risk factors such as age, sex, smoking, hypertension, dyslipidemia, etc. Our study also showed same finding. In our study, there was also significantly higher CAIMT in hyperuricemic CRF patients than non-hyperuricemic CRF patients and healthy controls. So, aggressive treatment of hyperuricemia may halt or slow the progression of atherosclerosis in CRF patients.
In concordance with “Normative aging study” [25], Elsayed et al [26], Tavil Y et al [2] our study also demonstrated that the hyperuricemia predicts the development of hypertension. So, controls of hyperuricemia can control hypertension in CRF patients and halt or slow the further progression of atherosclerosis.
Tseng et al [27] and Fukui et al [28] documented that hyperuricemia was associated with increased urinary albumin excretion. But Obermayr RP et al [10] reported that there was lack of relationship between SUA and proteinuria. Our study showed that albuminuria was significantly higher in hyperuricemic CRF patients than non-hyperuricemic CRF patients. So, controls of hyperuricemia can halt or slow the further progression of atherosclerosis by reducing albuminuria in CRF patients.
Viazzi F et al [29], Dehghan A et al [30] and Wiik BP et al [31] reported that hyperuricemia predict development of new onset diabetes. Our study also showed that Hyperuricemic CRF patients had significantly higher incidence of diabetes than CRF patients without hyperuricemia. So, controls of hyperuricemia can halt or slow the further progression of atherosclerosis by reducing development of new onset diabetes in CRF patients.
There were some limitations in this study because [1] premature atherosclerosis, serum homocysteine, lipoprotein (a), physical activity, atherogenic diet, pro-inflammatory factors and pro-thrombotic factors could not be examined due to the limitations of budget and study design, [2] small numbers of participants were included.
Conclusions
Hyperuricemia is independently responsible for increased thickening of carotid artery intima media thickness and also associated with higher level of albuminuria, and higher prevalence of hypertension and diabetes in CRF patients. So, aggressive control of hyperuricemia may halt or slow the progression of carotid artery intima media thickening in CRF patients. There is no difference of GFR between CRF patients with hyperuricemia and without hyperuricemia.
| References | ▴Top |
- Baroncini LA, de Oliveira A, Vidal EA, Franca GJ, Stahlke PS, Alessi A, Precoma DB. Appropriateness of carotid plaque and intima-media thickness assessment in routine clinical practice. Cardiovasc Ultrasound. 2008;6:52.
pubmed - Tavil Y, Kaya MG, Oktar SO, Sen N, Okyay K, Yazici HU, Cengel A. Uric acid level and its association with carotid intima-media thickness in patients with hypertension. Atherosclerosis. 2008;197(1):159-163.
pubmed doi - Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811-1821.
pubmed doi - Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53(5):796-803.
pubmed doi - Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;62(2):170-180.
pubmed - De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
pubmed - Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59(1):109-116.
pubmed doi - Savoiu G, Serban C, Novanu L, Mladinescu F, Gaita D, Duicu OM, et al. The role of hyperuricemia in endothelial dysfunction induced by hypertension. Romanian J. Biophys 2008;18:329-336.
- Viazzi F, Parodi D, Leoncini G, Parodi A, Falqui V, Ratto E, Vettoretti S, et al. Serum uric acid and target organ damage in primary hypertension. Hypertension. 2005;45(5):991-996.
pubmed doi - Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19(12):2407-2413.
pubmed doi - Serban C, Dragan S, Christodorescu R, Susan L, Mozos I, Caraba A, et al. Association of Serum Uric levels and Carotid Intima Thickness with the Numbers of Organ Damaged in Hypertensive Patients. International Journal of Collaborative Research on Internal Medicine & Public Health 2011; 3:8-16.
- The JNC 7 Report. JAMA. 2003; 21:1011-53.
- Bargman JM, Skorecki K. Chronic Kidney Disease. Harrison’s Principals of Internal Medicine, 17th edition, mcgraw-hill companies, 2008; 2: 1761- 1771.
- Lewis JB, Neilson EG. Glomerular Diseases. Harrison’s Principals of Internal Medicine, 18th edition, mcgraw-hill companies, 2008; glomerular diseases; 2: 2334-2354.
- Hoseini VN, Taziki O. Relationship between Microalbuminuria and Severity of Coronary Artery Disease in Non-Diabetic Patients. Iran Cardiovasc Res J 2008; 4: 234-237.
- Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Relation between serum uric acid and carotid intima-media thickness in healthy postmenopausal women. Intern Emerg Med. 2007;2(1):19-23.
pubmed doi - Kawashima M, Wada K, Ohta H, Terawaki H, Aizawa Y. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: a long-term retrospective cohort study. BMC Nephrol. 2011;12:31.
pubmed - Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
pubmed doi - Leskinen Y, Lehtimaki T, Loimaala A, Lautamatti V, Kallio T, Huhtala H, Salenius JP, et al. Carotid atherosclerosis in chronic renal failure-the central role of increased plaque burden. Atherosclerosis. 2003;171(2):295-302.
pubmed doi - Sahoo R, Krishna MV, Subrahmaniyan DK, Dutta TK, Elangovan S. Common carotid intima-media thickness in acute ischemic stroke: A case control study. Neurol India. 2009;57(5):627-630.
pubmed doi - Benedetto FA, Mallamaci F, Tripepi G, Zoccali C. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12(11):2458-2464.
pubmed - Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279(2):119-124.
pubmed doi - Kawamoto R, Tomita H, Oka Y, Kodama A, Ohtsuka N, Kamitani A. Association between uric acid and carotid atherosclerosis in elderly persons. Intern Med. 2005;44(8):787-793.
pubmed doi - Neogi T, Ellison RC, Hunt S, Terkeltaub R, Felson DT, Zhang Y. Serum uric acid is associated with carotid plaques: the National Heart, Lung, and Blood Institute Family Heart Study. J Rheumatol. 2009;36(2):378-384.
pubmed - Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, Weiss ST, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
pubmed doi - Elsayed AS, Mostafa MM, Abdelkhalik A, Eldeeb MEA, Abdulgani MS. Hyperuricemia and its association with carotid intima-media thickness in hypertensive and non hypertensive patients. Journal of the Saudi Heart Association 2010; 22: 19-23.
- Tseng CH. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 2005;68(2):796-801.
pubmed doi - Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, Kadono M, et al. Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism. 2008;57(5):625-629.
pubmed doi - Viazzi F, Leoncini G, Vercelli M, Deferrari G, Pontremoli R. Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: the MAGIC study. Diabetes Care. 2011;34(1):126-128.
pubmed doi - Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31(2):361-362.
pubmed doi - Wiik BP, Larstorp AC, Hoieggen A, Kjeldsen SE, Olsen MH, Ibsen H, Lindholm L, et al. Serum uric acid is associated with new-onset diabetes in hypertensive patients with left ventricular hypertrophy: The LIFE Study. Am J Hypertens. 2010;23(8):845-851.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
