| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Case Report
Volume 5, Number 1, March 2016, pages 11-15
Acute Renal Infarction Pathogenesis and Atrial Fibrillation: Case Report and Literature Review
Abdulwahab Hritania, Francois Jo-Hoya, Anwer Siddiqib, Candice Baldeoa, Andreea Poenariuc, d
aInternal Medicine Department, University of Florida, Jacksonville, FL, USA
bPathology Department, University of Florida, Jacksonville, FL, USA
cNephrology Division, Internal Medicine Department, University of Florida, Jacksonville, FL, USA
dCorresponding Author: Andreea Poenariu, Nephrology Division, Internal Medicine Department, University of Florida, 655 West 8th Street, Jacksonville, FL, USA
Manuscript accepted for publication October 07, 2015
Short title: Acute Renal Infarction Pathogenesis
doi: http://dx.doi.org/10.14740/wjnu242e
| Abstract | ▴Top |
Kidney infarction is an uncommon thromboembolic complication of atrial fibrillation (AF). Diagnosis of this condition can be challenging due to its rarity and the fact that its presentation is associated with a multitude of other pathologies. No treatment guidelines have been established thus far; however, multiple modalities have been employed, including systemic thrombolytics, intra-arterial thrombolytics and anticoagulation. In this article, we thought to review acute renal infarction pathogenesis with a focus on AF as an important etiology.
Keywords: Acute renal infarction; Atrial fibrillation; Pathogenesis
| Introduction | ▴Top |
Once thought to be a rare disease, acute renal infarction (ARI) is now being diagnosed more frequently due to the increased prevalence of patients with atrial fibrillation (AF) who have an increased risk for thromboembolic phenomenon. It is still an under-diagnosed cause of kidney dysfunction and often missed because its symptoms are misleading and may mimic renal colic, pyelonephritis or other conditions [1, 2]. Hence, early diagnosis is important, as renal function can be restored with revascularization of the occluded vessel [3].
| Case Report | ▴Top |
A 71-year-old Caucasian woman with a past medical history of hypertension and peripheral arterial disease presented to the hospital complaining of right-sided flank pain and hematuria. The patient did not have a history of AF. Her flank pain started suddenly 1 day prior to admission and was associated with nausea, vomiting and abdominal distension. She denied dysuria or increase in urinary frequency. Her home medications were aspirin, metoprolol twice daily and rosuvastatin.
On presentation, her blood pressure was 165/77 mm Hg and her heart rate was 67. On examination, she had a pulse with a regular rate and rhythm, no cardiac murmurs or gallops, but had right upper quadrant tenderness in addition to bilateral costovertebral angle tenderness. The rest of her physical examination was only significant for diminished lower extremity pulses.
Her initial evaluation showed a normal TSH level, a microscopic hematuria with few RBCs in the urine and a creatinine of 1.26 mg/dL up from her baseline of 0.72 mg/dL. Her initial electrocardiogram (EKG) showed unchanged left bundle branch block with normal sinus rhythm.
She had a non-contrast abdominal computed tomography (CT) scan that showed right-sided kidney infarct with small left-sided kidney infarcts (Fig. 1). A CT angiogram of the chest and abdomen revealed no aortic pathology but did confirm bilateral kidney infarcts. She had a transthoracic echo that showed moderate left atrial enlargement with no clot and normal left ventricular ejection fraction.
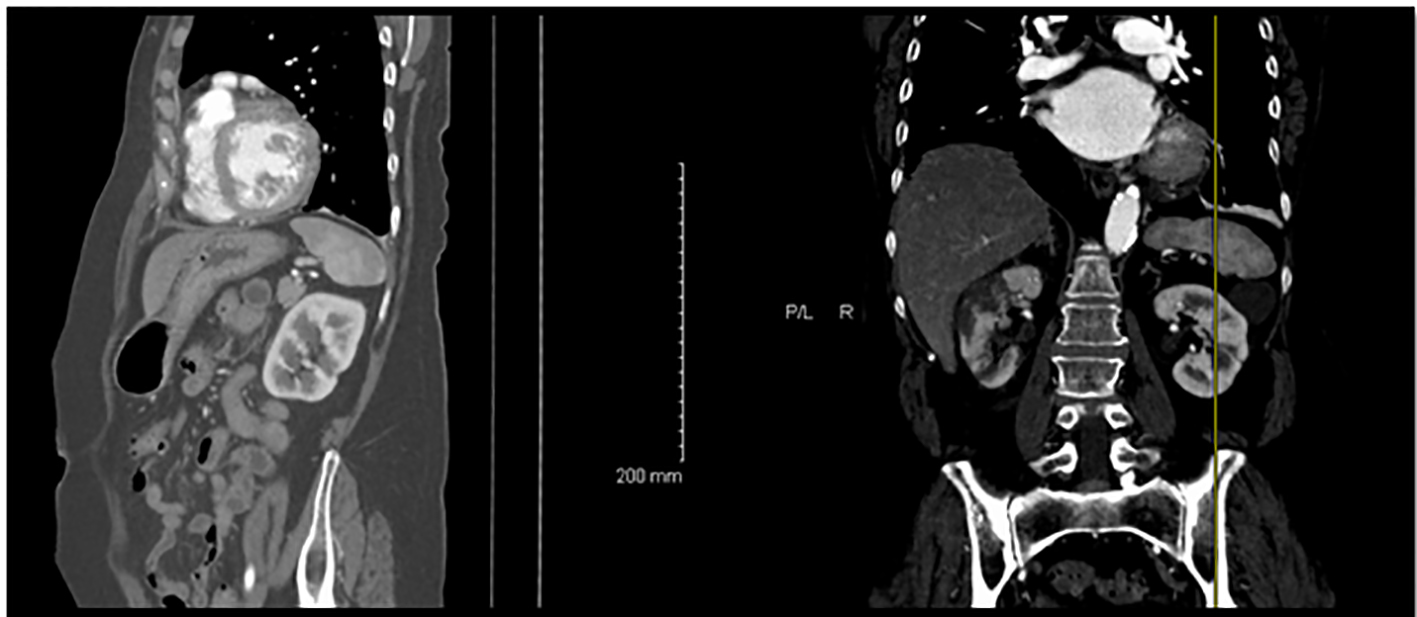 Click for large image | Figure 1. CT scan of the abdomen showing bilateral kidney infarctions. |
On the second day of hospitalization, her telemetry monitor showed an abnormal rhythm suggesting AF that was confirmed by an urgent EKG. A diagnosis of paroxysmal AF was made. Her CHA2DS2-VASc score was 6 which put her at a very high risk for thromboembolic events. The patient was anticoagulated appropriately with intravenous heparin in addition to warfarin and she was also continued on a beta-blocker regimen for rate control of her AF. Because of high CHA2DS2-VASc score, she was on lifelong anticoagulation.
The patient’s creatinine improved and went back to the baseline of 0.8 within less than a week.
| Discussion | ▴Top |
Renal vasculature anatomy
The abdominal aorta gives rise to the renal arteries at the levels of L1-L2. In the majority of individuals, the renal artery gives off branches that become the anterior and posterior divisions, and inside the hilum the anterior renal artery will give rise to the apical, anterior and inferior segmental branches. The segmental vessels branch deeper into the renal parenchyma to finally reach the capillaries and glomeruli [4] (Fig. 2). It is in fact believed that the renal circulation is the final resting place for up to 2.3% of systemic arterial embolizations [5] (Figs. 3-5).
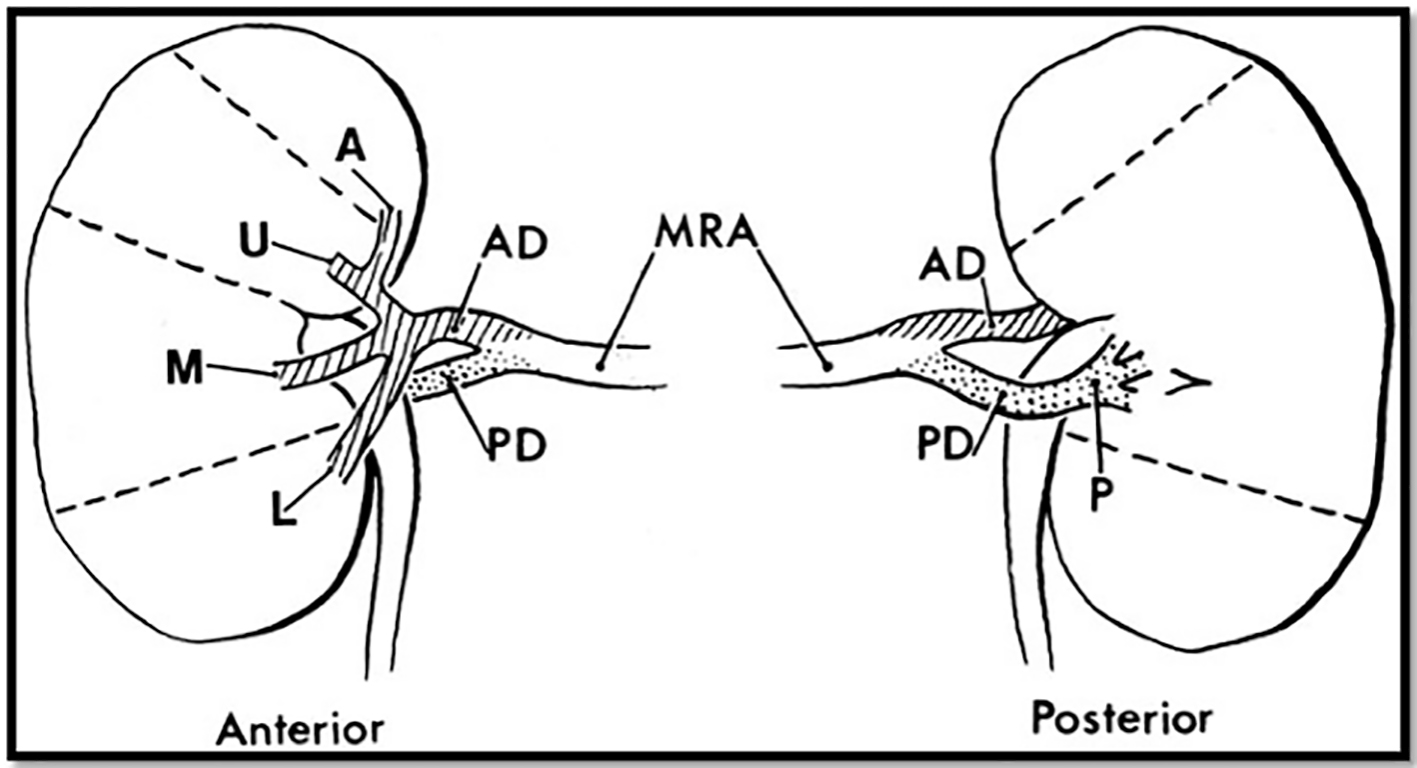 Click for large image | Figure 2. A diagram of the most common pattern of arterial supply to the kidneys demonstrating the main renal artery, anterior and posterior branches, and five segmental arteries. MRA: main renal artery; PD: posterior division; AD: anterior division. Segmental arteries are indicated by A (apical), U (upper), M (middle), L (lower), and P (posterior). Modified from Graves FT. The anatomy of the intrarenal arteries and its application to segmental resection of the kidney. Br J Surg 1954;42:132. |
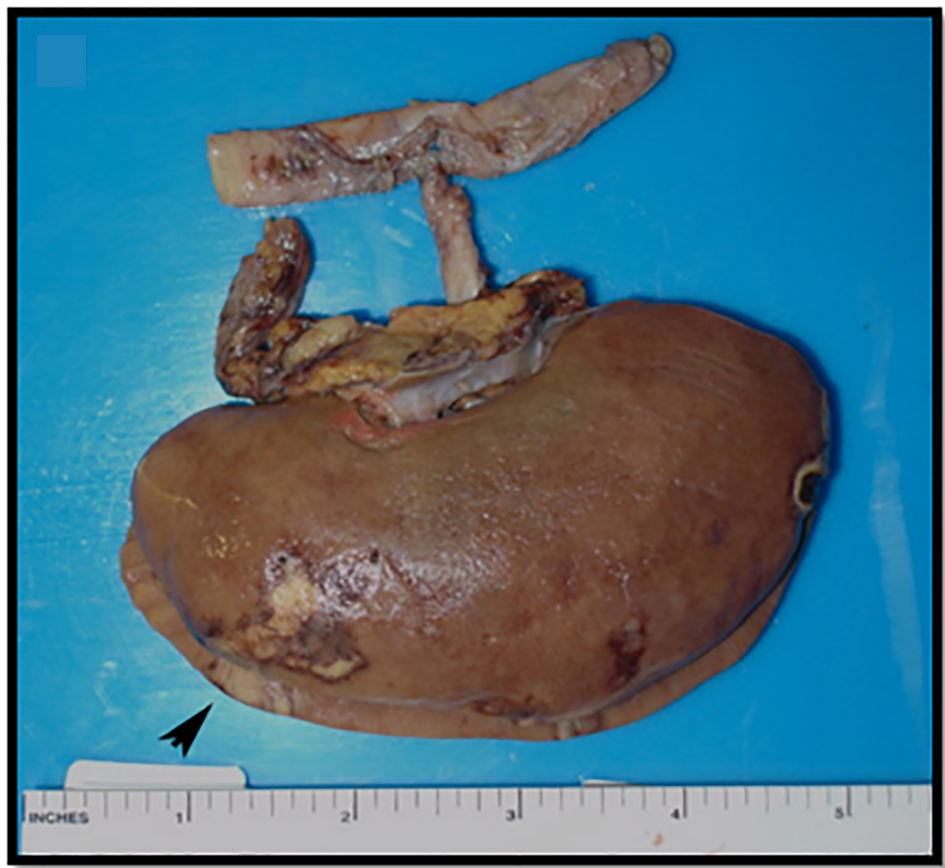 Click for large image | Figure 3. An allograft kidney from a 58-year-old obese man with a past medical history of hypertension and diabetes mellitus, who expired due to acute myocardial infarction and was found to have ARI on autopsy. |
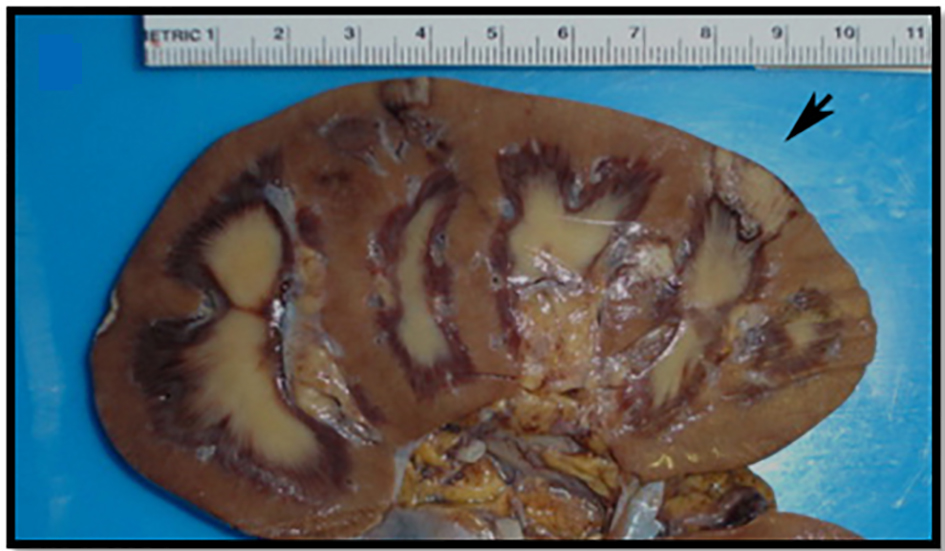 Click for large image | Figure 4. Gross image of the bivalved kidney with cortical infarcts secondary to arterial thrombus, largest infarct (black arrow) measuring 5.0 cm. |
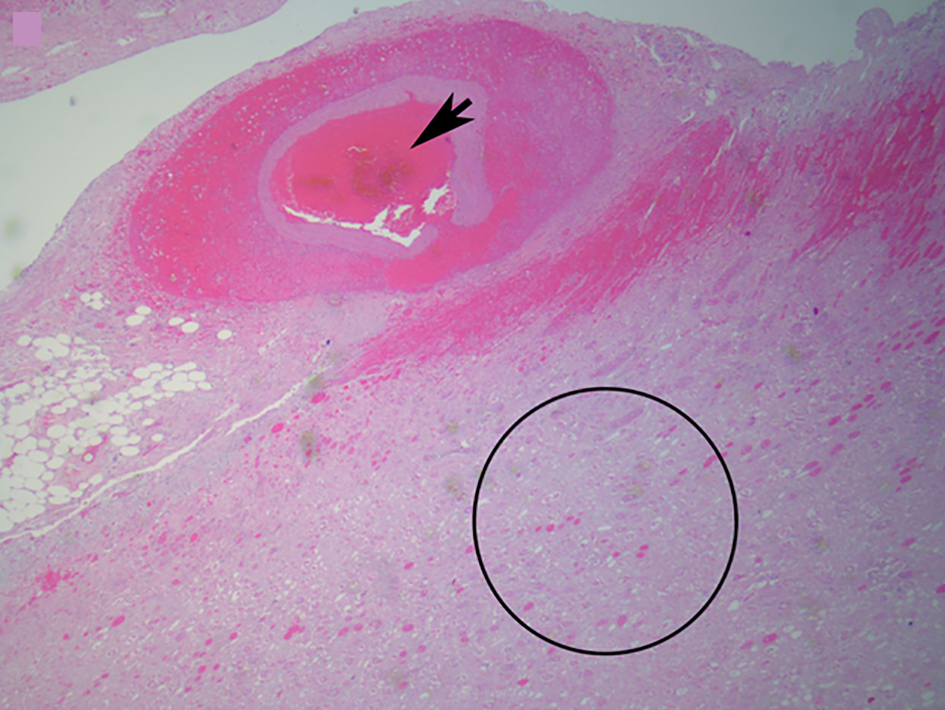 Click for large image | Figure 5. Microscopic image of an arterial thrombus showing a vessel completely occluded by the thrombus (black arrow) and the resulting renal infarct (in and around circle) (from Fig. 4) (H&E, × 2.5). |
ARI presentation and diagnosis
Renal infarction is often misdiagnosed as many other disorders including renal calculi and acute pyelonephritis present similarly and thus its presentation could be misleading [2].
Most patients with ARI are in their 60s and present with flank pain and hematuria with no predominance towards the right or left kidney. Acutely elevated blood pressure is thought to be secondary to renin-angiotensin-aldosterone related mechanisms. Other signs and symptoms seen are fever, nausea and vomiting. On the other hand, kidney infarctions may be completely asymptomatic and detected incidentally on a CT scan [1, 6, 7]. It has been estimated that renal infarction has an incidence of 1.4% based on autopsy series [8].
Elevations of white cell counts may be seen. Other serum markers have been found to be inconsistently elevated and include alkaline phosphatase, fibrinogen, C-reactive protein and aspartate aminotransferases. The most sensitive marker is serum lactate dehydrogenase, but it however is not specific [1, 9]. Renal angiography can virtually diagnose all cases but computed tomography (CT) imaging is key to diagnosing renal artery occlusion and has good sensitivity in detecting renal infarction [10].
ARI pathogenesis
There are several mechanisms for renal infarction with the thromboembolic phenomenon being the most common. This and other etiologies are discussed below.
Thromboembolic phenomenon
AF, previous embolic phenomenon, ischemic and valvular diseases put the patient at an increased risk for kidney infraction from a thromboembolic phenomenon [11]. The embolic pathogenesis involves the occlusion of a renal artery or branch vessel by cholesterol or blood clots [12, 13]. AF is the most common etiology for kidney infarction and the most important cause of thromboembolic phenomena [8].
AF is one of the most common arrhythmias accounting for more than 467,000 admissions annually in the United States. It has been estimated that 2% of Americans less than 65 years old and up to 9% older than 65 have AF. AF increases the risk of stroke and/or peripheral thromboembolism due to thrombi formation in the atrium and especially in the left atrial appendage. Thrombus formation in AF is classically attributed to blood flow stasis but it is now becoming apparent that thrombogenesis in AF is multifactorial. Loss of coordinated left atrial systole along with atrial dilation leads to atrial blood flow stasis. Anatomical changes seen in AF including endocardial denudation and even edematous and fibrinous changes may also play a role. Increased levels of prothrombin, prothrombin fragments 1 and 2, tissue factor and vWF are seen and there has been recent interest in the roles of IL-6 and growth factors (e.g. VGEF) as contributors to thrombus formation. Both IL-6 and VGEF can increase tissue factor production and are both increased in AF [14].
The CHA2DS2-VASc score is used in non-valvular AF to calculate the yearly stroke risk and is composed of congestive heart failure (one point), hypertension (one point), age ≥ 75 (two points), diabetes (one point), prior stroke, transient ischemic attack (TIA) or thromboembolism (two points), vascular disease (one point), age 65 - 74 (one point), and female sex (one point). Increase in a patient’s CHA2DS2-VASc score is associated with a considerably high incidence of yearly thromboembolic complications [14].
Korzets and colleagues evaluated 11 patients admitted with kidney infarctions between the years of 1997 and 2000. The prevalence in that review was thought to be 0.007% in one center with 60% of the cases identifying AF as the cause. In all the cases studied, the diagnosis was delayed because ARI was not initially part of the differential diagnosis. Ten of the 11 patients with ARI had recovery of their renal function [7].
Hazanov and colleagues described 44 patients with ARI between 1948 and 2002, among which 40 patients (90%) had a history of AF and four (10%) presented with AF with rapid ventricular response. Two (4%) of these patients were on warfarin and had therapeutic INR and yet they suffered ARI. The outcome for the renal function was available in 38 patients. Sixty-one percent had a normal kidney function on follow-up, while 18% and 8% ended up with renal failure (creatinine > 2 mg/dL) and end-stage renal disease, respectively. Two (4%) of these case had bilateral kidney involvement [15].
In a Danish study conducted by Frost and colleagues, 14,917 men and 14,945 women diagnosed with AF were followed up from diagnosis to first thromboembolic event, death or to the end of 1993. From this group, 2% were found to develop renal artery thromboembolic events [16].
An emergency department retrospective study done by Antopolsky and colleagues looked at 38 patients seen in the ED that were diagnosed with renal infarction during a 10-year period. Patients in whom infarction was due to trauma, coronary PCI, recent vascular surgery and those diagnosed after being seen in the ED were excluded. Of those included, AF was the most common etiology (n = 14, 37%). Other causes included hypercoagulation (n = 6, 16%), endocarditis (n = 3, 8%), others (n = 10, 26%), and unknown (n = 5, 13%) [10].
Hereditary and acquired clotting factors diathesis
Hypercoagulable disorders are a rare cause of kidney infarction. Amongst these antiphospholipid syndrome (APS), antithrombin III deficiency, factor V Leiden, protein C and S deficiencies, hyperhomocysteinemia and polycythemia vera play an important role [17, 18]. APS can cause both arterial and venous thrombotic events and while these events mostly involve lower limb deep veins and cerebral arteries, the renal circulation can also be targeted. Renal infarction can be a sequela of this and may be due to renal artery thrombosis or emboli from cardiac or other arterial sources [19]. The rarity of antithrombin III deficiency as a causative factor of ARI can be demonstrated as there was only one case report found of a 47-year-old male with antithrombin III deficiency and heterozygosity for prothrombin (G20210A) gene mutation who presented with bilateral renal infarcts [20].
Vessel anomalies
Fibro-muscular dysplasia (FMD) and renal artery aneurysm have been reported to cause kidney infarction. It has been reported in an otherwise healthy individual who presented with acute bilateral flank pain that ARI and bilateral FMD were found. In such cases, treatment is with stent placement leading to a complete resolution of kidney function [21].
Trauma
Kidney infarction has been reported with penetrating and blunt trauma to the abdomen with complete recovery of kidney function after conservative therapy with anticoagulation. The kidney dysfunction secondary to trauma can be divided into five grades, with grade 5 being the worst. The kidney injury secondary to trauma usually occurs when there is an intimal injury and thrombus formation in the renal artery. Sometimes kidney injury could be caused by severe retroperitoneal hemorrhage. Severe renal artery spasm causing decreased renal perfusion was reported with trauma as well [9, 22].
Idiopathic
Idiopathic etiology is an important cause for ARI. In some case series, it has been estimated that up to 60% of kidney infarction cases were idiopathic. These patients were younger and more likely to be cigarette smokers. However, this idiopathic etiology could have been overestimated as there are a significant number of these patients with a hypercoagulable state like hyperhomocysteinemia, antithrombin deficiency and protein S and C deficiency [18].
Less common causes
Kidney infarction has been reported with the use of cocaine and was attributed to cocaine-induced vasoconstriction, oxidative stress and increased atherogenicity [23]. Kidney infarction was also reported with mucormycosis infection when the kidney gets involved with the infection [18].
Treatment
Even though treatment guidelines have not been established, conservative therapy with thrombolysis or anticoagulation, as in our case, appears to be effective. Similarly to our case where creatinine returned to baseline within several days, recovery of renal function is achieved in most cases of renal infarction. This underlies the importance of always keeping ARI on the differential diagnosis list when a patient presents with flank pain and hematuria due to the need for prompt identification and treatment.
Conflict of Interest
The authors of this manuscript declare no conflict of interest.
| References | ▴Top |
- Bouassida K, Hmida W, Zairi A, Hidoussi A, Jaidane M, Slama A, Sorba NB, et al. Bilateral renal infarction following atrial fibrillation and thromboembolism and presenting as acute abdominal pain: a case report. J Med Case Rep. 2012;6:153.
doi pubmed - Huang CC, Kao WF, Yen DH, Huang HH, Huang CI, Lee CH. Renal infarction without hematuria: two case reports. J Emerg Med. 2006;30(1):57-61.
doi pubmed - ANA MARIA GOMEZ ARA1, et al. Acute renal infarction: an underdiagnosed cause of abdominal pain. Emergencias. 2010;22:117-119.
- Stephens FD, Smith ED, Hutson JM. Ureterovascular hydronephrosis and the aberrant renal vessels. In: Congenital Anomalies of the Kidney, Urinary and Genital Tracts. 2nd. New York: Informa Health Care; 2002:275-280.
- Goldberg G. Renal infarction. Ann Emerg Med. 1985;14(6):611-614.
doi - Bande D, Abbara S, Kalva SP. Acute renal infarction secondary to calcific embolus from mitral annular calcification. Cardiovasc Intervent Radiol. 2011;34(3):647-649.
doi pubmed - Korzets Z, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002;4(10):781-784.
pubmed - Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, Halimi JM, Karras A, et al. Acute renal infarction: a case series. Clin J Am Soc Nephrol. 2013;8(3):392-398.
doi pubmed - Ucar A, Yahyayev A, Agayev A, Yanar F, Bakan S, Bulakci M, Akman T, et al. Severe spasm of the renal artery after blunt abdominal trauma simulating end-organ infarction. Case Rep Med. 2010;2010:207152.
doi - Antopolsky M, Simanovsky N, Stalnikowicz R, Salameh S, Hiller N. Renal infarction in the ED: 10-year experience and review of the literature. Am J Emerg Med. 2012;30(7):1055-1060.
doi pubmed - Lin HC, Shih PM, Chang TH, Ke HL, Wu WJ, Huang CH. Successful thrombolytic therapy for bilateral renal infarction: a case report. Kaohsiung J Med Sci. 2006;22(8):410-414.
doi - Demuynck F, Blanpain S, Brochart C, Morvan J, Boukadoum N, Fuks D, Kocheida M, et al. [Renal infarction: a rare manifestation of patent foramen ovale]. J Radiol. 2008;89(9 Pt 1):1101-1104.
doi - Manfredini R, La Cecilia O, Ughi G, Kuwornu H, Bressan S, Regoli F, Orzincolo C, et al. Renal infarction: an uncommon mimic presenting with flank pain. Am J Emerg Med. 2000;18(3):325-327.
doi - Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373(9658):155-166.
doi - Hazanov N, Somin M, Attali M, Beilinson N, Thaler M, Mouallem M, Maor Y, et al. Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine (Baltimore). 2004;83(5):292-299.
doi - Frost L, Engholm G, Johnsen S, Moller H, Henneberg EW, Husted S. Incident thromboembolism in the aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from the hospital with a diagnosis of atrial fibrillation. Arch Intern Med. 2001;161(2):272-276.
doi pubmed - Saeed K. Renal infarction. Int J Nephrol Renovasc Dis. 2012;5(119-123.
- Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S. Idiopathic renal infarction. Am J Med. 2006;119(4):356 e359-312.
- Sciascia S, Cuadrado MJ, Khamashta M, Roccatello D. Renal involvement in antiphospholipid syndrome. Nat Rev Nephrol. 2014;10(5):279-289.
doi pubmed - Wiles KS, Hastings L, Muthuppalaniappan VM, Hanif M, Abeygunasekara S. Bilateral renal artery thrombosis in inherited thrombophilia: a rare cause of acute kidney injury. Int J Nephrol Renovasc Dis. 2014;7:35-38.
doi pubmed - Dursun B, Yagci B, Batmazoglu M, Demiray G. Bilateral renal infarctions complicating fibromuscular dysplasia of renal arteries in a young male. Scand J Urol Nephrol. 2012;46(1):73-77.
doi pubmed - Abdur Baig, et al. Acute Renal Infarction: An Underdiagnosed Disorder. J Med Cases. 2012;3(3):197-200.
doi - Bemanian S, Motallebi M, Nosrati SM. Cocaine-induced renal infarction: report of a case and review of the literature. BMC Nephrol. 2005;6:10.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
