| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Case Report
Volume 7, Number 3-4, November 2018, pages 73-77
A Case of Myeloma Kidney With Glomerular C3 Deposition
Asif Khana, c, Khine Lamb, Suzanne El-Sayeghb, Elie El-Charabatyb
aDepartment of Medicine, Staten Island University Hospital, Staten Island, NY 10305, USA
bDepartment of Nephrology, Staten Island University Hospital, Staten Island, NY 10305, USA
cCorresponding Author: Asif Khan, Department of Medicine, Staten Island University Hospital, 475 Seaview Avenue, Staten Island, NY 10305, USA
Manuscript submitted July 1, 2018, accepted August 27, 2018
Short title: Myeloma Kidney With Glomerular C3 Deposition
doi: https://doi.org/10.14740/wjnu359w
| Abstract | ▴Top |
C3 glomerulonephritis is rare form of membranoproliferative glomerulonephritis, which result from defects in complement regulatory proteins that promotes excessive activation of alternative complement pathway. Kidney disease is common complication of multiple myeloma (MM). Most common renal complications in MM include monoclonal immunoglobulin deposition disease and myeloma cast nephropathy. Moreover, monoclonal Ig, through the interference of the complement alternative pathway has been shown to play the synergistic role towards renal damage. Up to 50% of MM patients present with renal impairment at diagnosis, 20% may present with acute kidney injury, and 10% require dialysis. In this case report, we describe a case of MM with cast nephropathy with mesangial staining for C3 consistent with C3 glomerulopathy, and the interrelationship between MM and complement system that leads to C3 glomerulopathy. A 59-year-old Trinidadian man with a 2-year history of hypertension presented with nausea and vomiting associated with a generalized weakness for the past 3 months. On admission laboratory results were as follows: hemoglobin 9.1 g/dL; red blood count 1.45 × 106/mm3; white cell count 3.2 × 103/mm3; platelet count 94 × 103/mm3; blood urea nitrogen 94 mg/dL; serum creatinine 10.47 mg/dL (patient had a baseline creatinine level of 1.5 mg/dL); sodium 130 mEq/L; potassium 6.2 mEq/L; bicarbonate 14 mEq/L; total protein 7.0 g/dL; albumin 2.9 g/dL; alkaline phosphatase 57; AST and ALT normal; lipase 102 U/L; urine analysis showed 2+ protein with bland urine sediment and microscopic hematuria (3 - 6/HPF). 24-h urine protein was 2 g/day. Renal ultrasound was significant for a right renal 0.6 cm cyst. The patient was admitted to ICU and was subsequently hemodialyzed due to worsening hyperkalemia and acute kidney injury. Serologies were notable for positive anti-dsDNA antibody and low levels of C3 (46 mg/dL) with normal C4 were observed. Immunofixation by electrophoresis showed free lambda. Serum plasma electrophoresis showed two M-spikes: Lambda light chains and IgG Lambda. A renal biopsy was performed and cast nephropathy was identified with mesangial staining for C3. Bone marrow biopsy was performed and showed CD 56-positive plasma cell myeloma. Patient was treated with Velcade, Cytoxan, and dexamethasone. The patient was subsequently discharged on chemotherapy and intermittent hemodialysis therapy. Follow-up evaluation of the alternative complement pathway showed normal activity level. This case illustrated myeloma kidney associated with mesangial C3 deposition in glomeruli and C3 hypocomplementemia. Our hypothesis is that in monoclonal gammopathy induced C3 glomerulopathy; paraprotein itself is acting as a trigger that excessively activates and dysregulates the AC pathway systemically. Thus, it is highly feasible to tailor the treatment to reduce the amount of paraproteins in C3 glomerulopathy associated with myeloma kidney, as opposed to conventional treatment for C3 glomerulopathy such as plasma exchange, rituximab/eculizumab, etc. Recognizing of the association between C3 GN and MM is important because it can be used as potential marker for hematological malignancy as well as the potential effective treatment for C3 G associated MM.
Keywords: Multiple myeloma; Myeloma kidney; Glomerular C3 deposition
| Introduction | ▴Top |
Multiple myeloma (MM) is a lymphoproliferative disorder characterized by the clonal proliferation of plasma or lymphoplasmacytic cells leading to the secretion of paraproteins: monoclonal immunoglobulin (Ig) or variable quantities of Ig fragments (monoclonal free light chain). An elevated free kappa/lambda ratio in serum outside the normal range (0.26 - 1.65) is attributed to the presence of monoclonal free light chains [1]. The monoclonal Igs are detected as a monoclonal band (M-band) on serum electrophoresis on serum and/or in the urine as Bence-Jones protein [2]. The light chains can precipitate in the proximal and distal tubules causing inflammation with secondary interstitial involvement [3]. Most common renal complications of MM include monoclonal immunoglobulin deposition disease and myeloma cast nephropathy [4]. Moreover, monoclonal Ig, through the interference of the complement alternative pathway (CAP) has been shown to play the synergistic role towards renal damage [5]. Up to 50% of MM patients present with renal impairment at diagnosis, 20% may present with acute kidney injury, and 10% require dialysis [6, 7].
The complement system
The complement cascade is initiated by three initial pathways: classical, lectin and alternative (Fig. 1) [8]. Activation of all three-complement pathways results in assembly of the membrane attack complex (MAC) on cell surfaces, and subsequent in cell lysis [9]. Classical complement pathway is triggered by antigen-antibody immune complexes. However, the alternative complement pathway is constantly activated “tick over” at a low rate. To limit damage to self, half of the complement proteins tightly regulate the system by both activator and inhibitor proteins such as complement factor H (CFH) and complement factor I (CFI) [10]. Mutations or autoantibodies such as C3 nephritic factor (C3Nef) against these complement regulatory proteins promote the erratic activity of the complement cascade, causing deposition of complement components on the wall of the glomerular capillaries causing tissue damage [11].
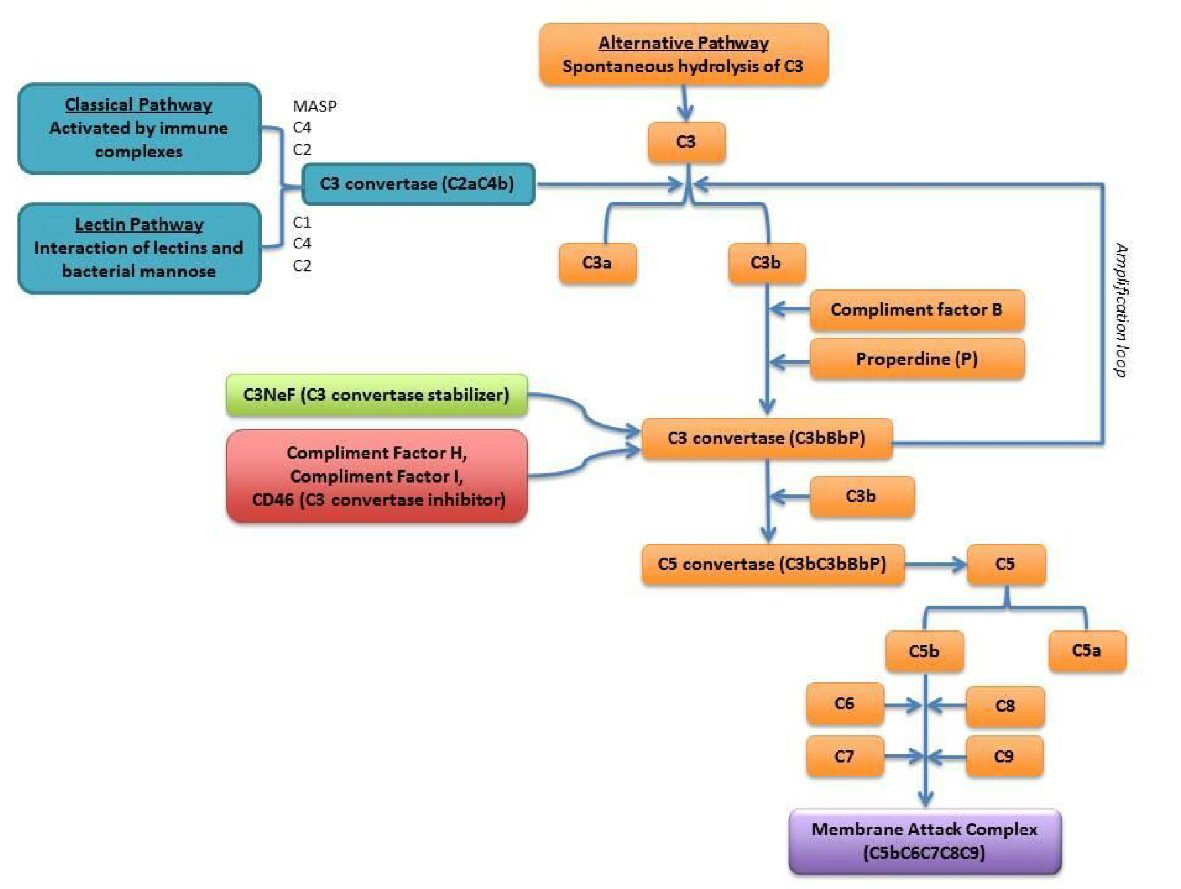 Click for large image | Figure 1. Scheme of the complement system: alternative complement pathway is outlined in orange [8]. |
C3 glomerulopathy
In C3 glomerulopathy, which is one of the complement-mediated types of membranoproliferative glomerulonephritis (MPGN), defects in complement regulatory proteins promote the excessive activation of the alternative pathway [12]. It can be due to either idiopathic or acquired, in which the latter is caused by acquired autoantibodies such as paraproteins that target the complement regulatory proteins involved in alternative pathway. The paraproteins acts as C3 nephritic factor stabilizing C3 convertase by preventing CFH mediated decay. This results in unregulated production of C3b, C5 convertase that ultimately lead to membrane attack complex formation. Other complement abnormality has also been identified as potential causes of C3 glomerulopathy, such as auto-antibodies to factor H, factor I, factor B and membrane cofactor protein [13].
In this case report, we describe a case of MM with cast nephropathy with mesangial staining for C3 consistent with C3 glomerulopathy, and the interrelationship between MM and complement system that leads to C3 glomerulopathy.
| Case Report | ▴Top |
A 59-year-old Trinidadian man with a 2-year history of hypertension (HTN) presented with nausea and vomiting associated with a generalized weakness for the past 3 months. The patient had recently hiked the Delaware River trail and reported having few insect bites without rash. He denied any fever or chills or any other symptoms. He does not take any medications and was placed on life style modification for his HTN management. As per patient, his laboratory results from a few months ago were within normal limits. He had no new concerns at the time of presentation.
At the time of admission, physical examination reveals a well-appearing afebrile male with a blood pressure of 137/77 mm Hg, a pulse of 103 bpm, with fairly normal physical findings. On admission laboratory results were as follows: hemoglobin 9.1 g/dL; red blood count 1.45 × 106/mm3; white cell count 3.2 × 103/mm3; platelet count 94 × 103/mm3; mean corpuscular volume 92 pg; blood urea nitrogen (BUN) 94 mg/dL; serum creatinine (Cr) 10.47 mg/dL (patient had a baseline creatinine level of 1.5 mg/dL); BUN/Cr 8.77; FeNa 8.7%; sodium 130 mEq/L; potassium 6.2 mEq/L; bicarbonate 14 mEq/L; total protein 7.0 (6 - 8) g/dL; albumin 2.9 g/dL; alkaline phosphatase 57; AST and ALT normal; lipase 102 U/L; urine analysis showed 2+ protein with bland urine sediment and microscopic hematuria (3 - 6/HPF). 24-h urine protein was 2 g/day. Renal ultrasound was significant for a right renal 0.6 cm cyst. Chest X-ray (CXR) was negative. Electrocardiogram (EKG) was normal and 2D echo was negative with an EF of 55% - 65%.
The patient was admitted to intensive care unit (ICU) for hyperkalemia with acute kidney injury (AKI) management. He received packed red blood cell transfusion and was consequently hemodialyzed. Serologies were notable for positive anti-dsDNA antibody and low levels of C3 (46 mg/dL) with normal C4 were observed. Test for rheumatoid factor, Smith Ab, and Sjogren Ab. Lyme, Babesiosis, HCV and HIV were negative. Anti-glomerular basement membrane antibody and cryoglobulins were negative. Based on serological finding lupus nephritis was suspected and renal biopsy as scheduled. Pancytopenia was blamed on SLE; however, patient did not have any cutaneous manifestations. Rheumatology was consulted and he was started on PO prednisone (60 mg/day).
Immunofixation by electrophoresis of both serum and urine showed free lambda (monoclonal free light chain) band present. Serum plasma electrophoresis showed two M-spikes: lambda light chains and IgG lambda. Free kappa/lambda ratio < 0.01 (0.26 - 1.65) with a free lambda serum of 3,799 (5.7 - 26.3). Urine electrophoresis showed urine total protein: 1,896 mg/L (50 - 250) with three monoclonal bands. Serum levels of IgG 2,500 mg/dL (694 - 1,618); IgA 20 mg/dL (68 - 378); Ig M 9 mg/dL (40 - 230). A renal biopsy was performed and cast nephropathy was identified with mesangial staining for C3 (Figs. 2-4).
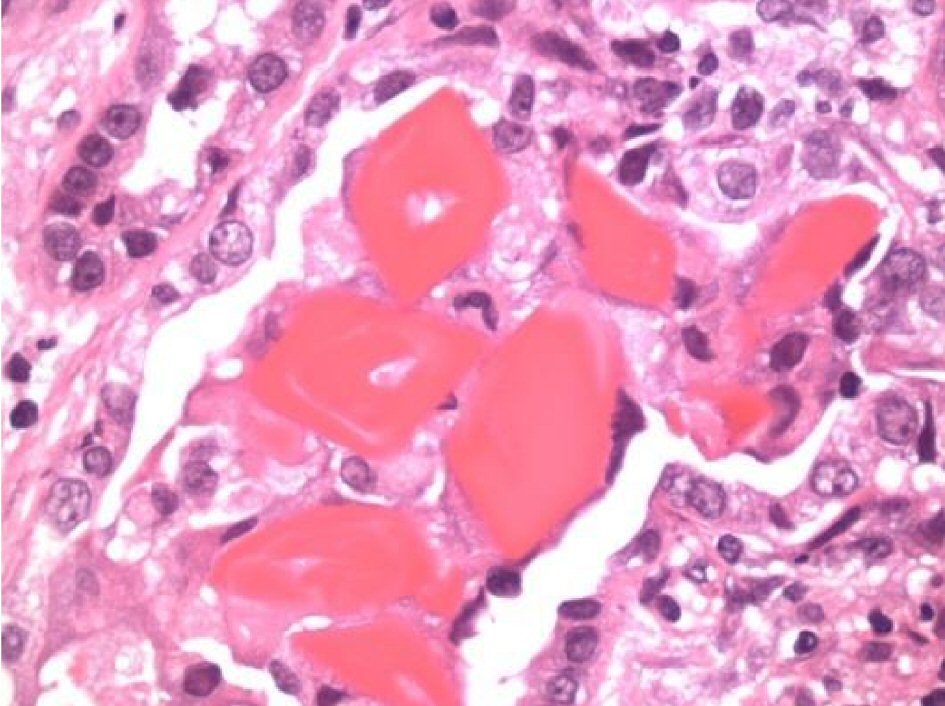 Click for large image | Figure 2. Under high magnification: atypical light chain fractured casts “myeloma kidney” with diffuse tubular injury and moderate tubular atrophy, interstitial fibrosis and diffuse interstitial inflammation by lymphocytes, monocytes, occasional plasma cells and scattered neutrophils. Moderate arteriosclerosis was noted. |
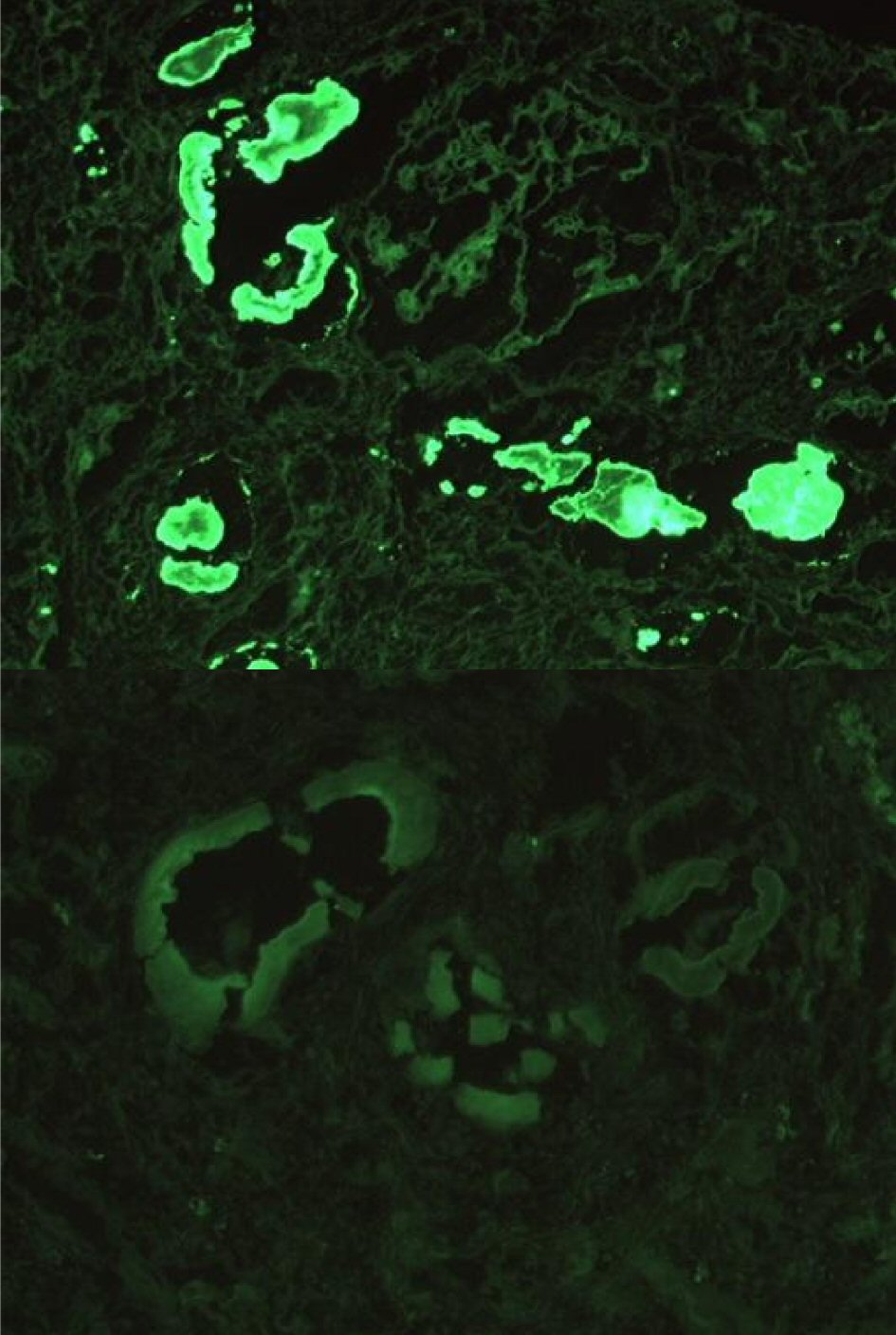 Click for large image | Figure 3. Immunofluorescence revealed 3+ staining for lambda in the distribution of the atypical casts (above) with weak granular mesangial staining for C3 (below). |
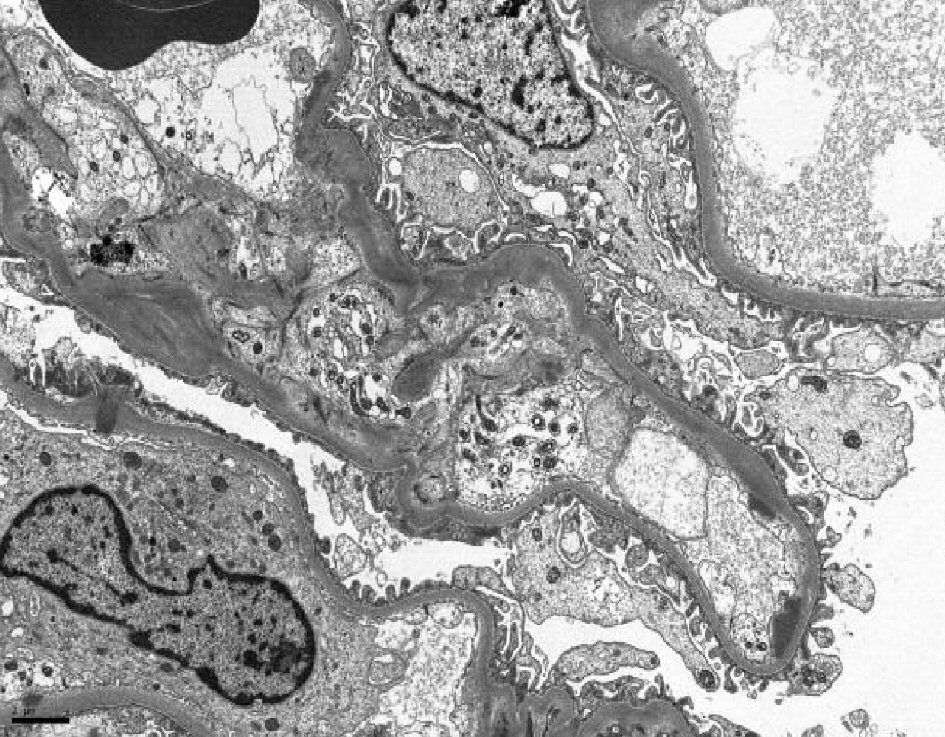 Click for large image | Figure 4. Electron microscopy showed scattered global mesangial and segmental subendothelial immune deposits. |
With the abnormal free light chain ratio, the presence of serum IgG κ light chain monoclonal band, and the findings on the kidney biopsy, additional tests for lymphoproliferative disease were undertaken. A bone marrow biopsy was performed and showed CD 56-positive (negative in normal plasma cell/plasma cell leukemia) plasma cell myeloma (42% IgG lambda). Flow cytometry was indicative of immunophenotypical aberrance; with κ light chain restricted plasma cells. A skeletal survey showed unremarkable results. Therefore, MM was diagnosed with IgG lambda cast nephropathy and end-stage renal disease on dialysis with pancytopenia.
He was treated with Velcade (bortezomib 3.5 mg), Cytoxan, and dexamethasone. He was also found to be vitamin B12 deficient and started on vitamin B12 shot weekly. SPEP status post chemotherapy showed two M-spikes. The patient was subsequently discharged on chemotherapy and intermittent hemodialysis therapy and was followed up in both the nephrology and hematology clinics. Evaluation of the alternative complement pathway showed normal activity level. At 4 months post-biopsy, the patient remained dependent on hemodialysis therapy and required multiple blood transfusions to maintain hemoglobin of 8 g/dL.
| Discussion | ▴Top |
We present the case of myeloma kidney associated with C3 hypocomplementemia and granular pattern mesangial C3 deposition in glomeruli as seem in C3 glomerulopathy. Although our patient did not exhibit the typical characteristics of MPGN on light microscopy, immunofluorescence (IF) does reveal characteristics of granular pattern of mesangial C3 deposition without any immunoglobulin deposits usually found in immune complex mediated diseases. EM also reveals global mesangial and segmental subendothelial immune deposits, as well as rare subepithelial deposits. Tubulointerstitial changes such as diffuse tubular injury, tubular atrophy, interstitial fibrosis and diffuse interstitial inflammation were also observed. One can argue that some of these findings can also be seen in post-strep GN. However, in post-strep GN, which is one of the immune-complex mediated diseases, the presence of immunoglobulin depositions is usually appreciated along with C3 depositions on IF in post-strep GN. The duration of the disease course and the persistent depression of C3 beyond 6 - 8 weeks are more common with C3 glomerulopathy.
It is important that we suspect and test for monoclonal gammopathy in patients present with C3 nephropathy. Although there have been the literatures that suggested the potential associations between monoclonal gammopathy and C3 glomerulopathy, it is still yet to be definitively established [14]. Our hypothesis is that in monoclonal gammopathy induced C3 glomerulopathy; paraprotein itself is acting as a trigger that excessively activates and dysregulates the AC pathway systemically. Thus, it is highly feasible to tailor the treatment to reduce the amount of paraproteins in C3 glomerulopathy associated with myeloma kidney, as opposed to conventional treatment for C3 glomerulopathy such as plasma exchange, rituximab/eculizumab, etc.
Conclusions
In conclusion, we report a rare case of MM light chain cast nephropathy associated with C3 glomerular deposits consistent with C3GN. The possible mechanism to which the abnormal monoclonal immunoglobulins triggering the excessive activation of alternative complement pathway contributing to the development of C3G is also proposed. The improvement of renal function with the reduction in proteinuria following chemotherapy in our MM patient further strengthens this argument. Currently there are no effective disease-specific treatments for C3G. Conventional treatments used in C3G are supportive measure with ACEI/ARBs, plasma exchange or humanized monoclonal antibodies such as eculizumab or rituximab. The association between C3G with hypocomplementemia and MM is rapidly gaining attention, because not only it can be a potential marker for underlying hematological malignancy [15], but also helps us find the potential effective treatment for C3G associated with MM.
Conflict of Interest
The authors of this manuscript have no conflict of interest to disclose.
Disclosure
None of the authors have financial support.
| References | ▴Top |
- Abraham GN, Waterhouse C. Evidence for defective immunoglobulin metabolism in severe renal insufficiency. Am J Med Sci. 1974;268(4):227-233.
doi - Wochner RD, Strober W, Waldmann TA. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967;126(2):207-221.
doi pubmed - Sanders PW, Herrera GA, Kirk KA, Old CW, Galla JH. Spectrum of glomerular and tubulointerstitial renal lesions associated with monotypical immunoglobulin light chain deposition. Lab Invest. 1991;64(4):527-537.
pubmed - Kowalewska J, Nicosia RF, Smith KD, Kats A, Alpers CE. Patterns of glomerular injury in kidneys infiltrated by lymphoplasmacytic neoplasms. Hum Pathol. 2011;42(6):896-903.
doi pubmed - Zand L, Kattah A, Fervenza FC, Smith RJ, Nasr SH, Zhang Y, Vrana JA, et al. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am J Kidney Dis. 2013;62(3):506-514.
doi pubmed - Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
doi pubmed - Gertz MA. Managing myeloma kidney. Ann Intern Med. 2005;143(11):835-837.
doi pubmed - Sandhu G, Bansal A, Ranade A, Jones J, Cortell S, Markowitz GS. C3 glomerulopathy masquerading as acute postinfectious glomerulonephritis. Am J Kidney Dis. 2012;60(6):1039-1043.
doi pubmed - Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729-740.
doi pubmed - Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis - a new look at an old entity. N Engl J Med. 2012;366(12):1119-1131.
doi pubmed - Habbig S, Mihatsch MJ, Heinen S, Beck B, Emmel M, Skerka C, Kirschfink M, et al. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75(11):1230-1234.
doi pubmed - Rabasco-Ruiz C, Huerta-Arroyo A, Caro-Espada J, Gutierrez-Martinez E, Praga-Terente M. C3 glomerulopathies. A new perspective on glomerular diseases. Nefrologia. 2013;33(2):164-170.
pubmed - Barbour S, Gill JS. Advances in the understanding of complement mediated glomerular disease: implications for recurrence in the transplant setting. Am J Transplant. 2015;15(2):312-319.
doi pubmed - Yin G, Cheng Z, Zeng CH, Liu ZH. C3 glomerulonephritis in multiple myeloma: A case report and literature review. Medicine (Baltimore). 2016;95(37):e4843.
doi pubmed - Cooper DL, Munday WR, Moeckel GW. C3 glomerulonephritis and plasma cell dyscrasia: expanding the etiologic spectrum. Biol Med. 2015;7:252.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
