| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Case Report
Volume 10, Number 1, September 2021, pages 15-17
From Hypokalemic Crisis to Sjogren’s Syndrome: A Case Report and Literature Review
Mansour Mbenguea, d, Cedric Ouanekponeb, Seynabou Diagnec, Abdou Nianga
aDepartment of Nephrology, Dalal Jamm University Hospital, Dakar, Senegal
bDepartment of Nephrology, Aristide Le Dantec University Hospital, Dakar, Senegal
cDepartment of Nephrology, Pikine Hospital, Dakar, Senegal
dCorresponding Author: Mansour Mbengue, Department of Nephrology, Dalal Jamm University Hospital, Dakar, Senegal
Manuscript submitted January 31, 2021, accepted February 11, 2021, published online September 28, 2021
Short title: From Hypokalemic Crisis to SS
doi: https://doi.org/10.14740/wjnu423
| Abstract | ▴Top |
Renal involvement occurs in approximately 5% of patients with Sjogren’s syndrome (SS). We report the case of a 20-year-old African woman who developed paraplegia secondary to hypokalemia. The diagnosis of renal tubular acidosis type 1 complicated by hypokalemia was made. After a search for the cause of renal tubular acidosis type 1, a diagnosis of primary SS was made. The patient received symptomatic treatment consisting of potassium chloride, sodium bicarbonate, hydration and a low protein diet. In terms of treatment, she was put on corticosteroid and hydroxychloroquine. The outcome was favorable with correction of acidosis and hypokalemia.
Keywords: Paraplegia; Hypokalemia; Renal tubular acidosis type 1; Sjogren’s syndrome; Corticosteroid
| Introduction | ▴Top |
Renal involvement occurs in approximately 5% of patients with Sjogren’s syndrome (SS) [1]. The disorders described are very heterogeneous according to the studies [1]. Three renal lesions predominate: chronic tubulointerstitial nephritis, tubulopathies and membranoproliferative glomerulonephritis [1]. We report the case of a patient who presented with distal renal tubular acidosis type 1 (DTA1) secondary to SS, discovered in the context of hypokalemic paralysis. We then provided an updated review of the pathophysiological mechanisms that may explain the link between DTA1 and SS.
| Case Report | ▴Top |
A 20-year-old African woman presented to Dalal Jamm Hospital for paralysis of four limbs. The physical examination showed paraplegia and arrhythmia on cardiac auscultation. Vital signs were significant for blood pressure 120/80 mm Hg, pulse 88 beats/min, temperature 36.6 °C, weight 69 kg and diuresis 3 L/24 h. This patient had a long history of hospitalization in the neurology department for paraplegia secondary to the hypokalemic crisis. She had no family history of kidney disease or sickle cell disease. The Schirmer test was positive. Laboratory investigations revealed serum: K+: 1.4 mmol/L, Na+: 134 mmol/L, Cl-: 113 mmol/L; urine studies: Na+: 250 mmol/24 h, K+: 53 mmol/24 h, Cl-: 254 mmol/24 h; urinary urea 5.2 g/24 h, urine creatinine 139 mg/L, glucose 0.99 g/L, urinary osmolarity 202.6 mmol/L, plasma osmolarity 290.3 mmol/L, the transtubular potassium gradient (TTKG) 5, serum magnesium 23 mg/L, alkaline reserves 15.69 mmol/L, the plasma anion gap 9.3, urine anion gap 10, urine pH 7.5, serum creatinine 7 mg/L, calcium 90 mg/L, phosphorus 25 mg/L and urine calcium 187 mg/24 h. Abdomen X-ray showed nephrocalcinosis (Fig. 1). The diagnosis of DTA1 complicated by hypokalemia was made. The electrocardiogram showed atrial extrasystoles. The hemogram showed hemoglobin 12.4 g/dL, white blood cells 4,080/mm3, lymphopenia at 900/mm3 and platelets 371,000/mm3. Thyroid-stimulating hormone (TSH) and C-reactive protein were normal. Electrophoresis of serum proteins showed polyclonal hypergammaglobulinemia. Anti-nuclear antibodies were positive at 380 with speckled fluorescence. The native anti-DNA antibodies were negative. The anti-SSA was positive. The anti-SSB was negative. The accessory salivary gland biopsy showed grade 4 lymphocytic sialadenitis from Chisholm and Mason (Fig. 2). We made the diagnosis of primary SS because she had a score according to 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome of 7 (The Schirmer test positive (score = 1), Labial salivary gland with focal lymphocytic sialadenitis, focus score of ≥ 1 foci/4 mm2 (score = 3), anti-SSA/Ro positive (score = 3)) and she had no exclusion criteria. Primary SS was identified as the cause of type 1 renal tubular acidosis. The patient received symptomatic treatment based on potassium chloride, sodium bicarbonate, hydration, a low protein diet and rich in fruits and vegetables. In terms of treatment, she was put on corticosteroid and hydroxychloroquine. The outcome was favorable with correction of acidosis and hypokalemia.
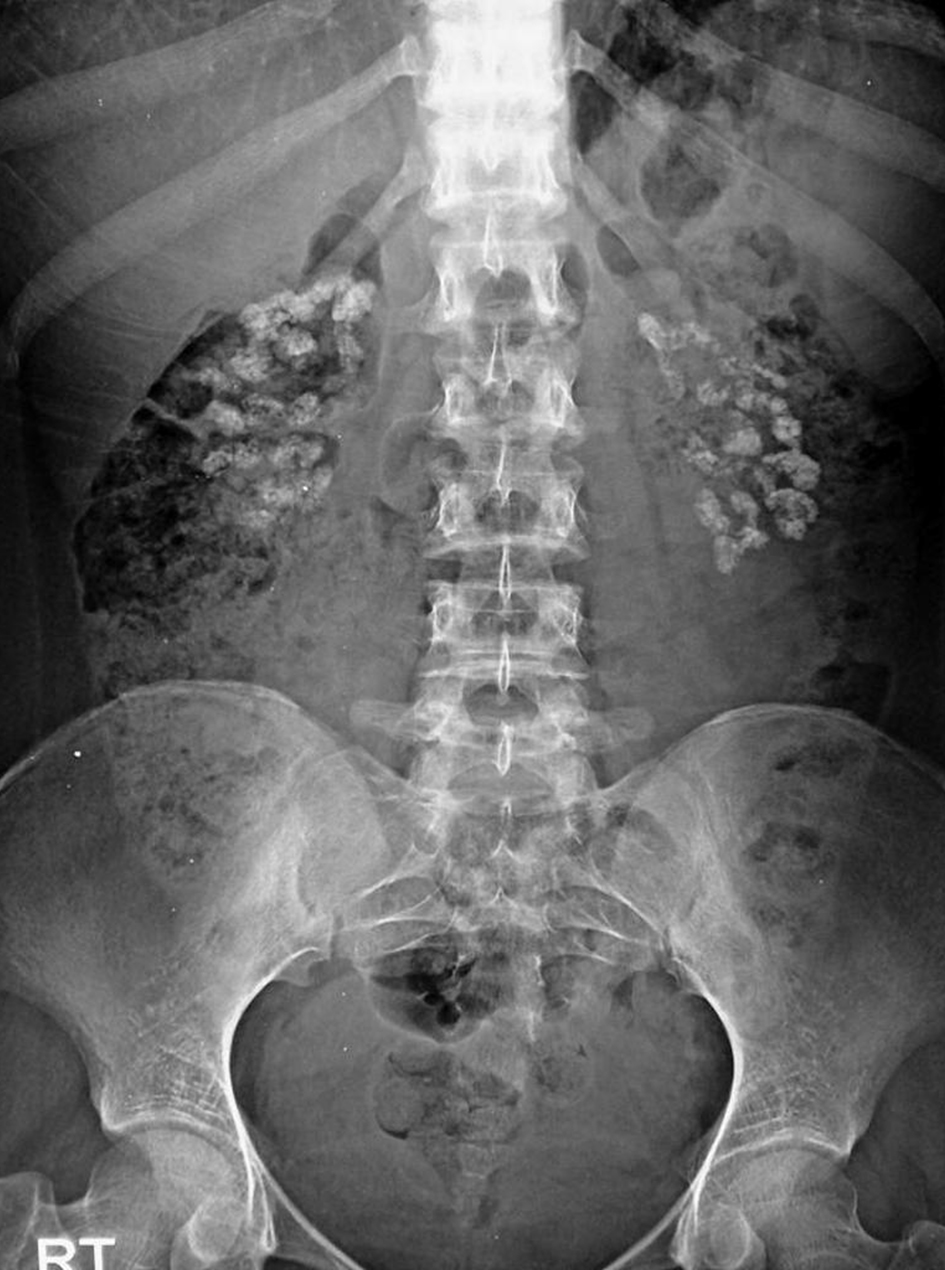 Click for large image | Figure 1. Abdomen X-ray showing nephrocalcinosis. |
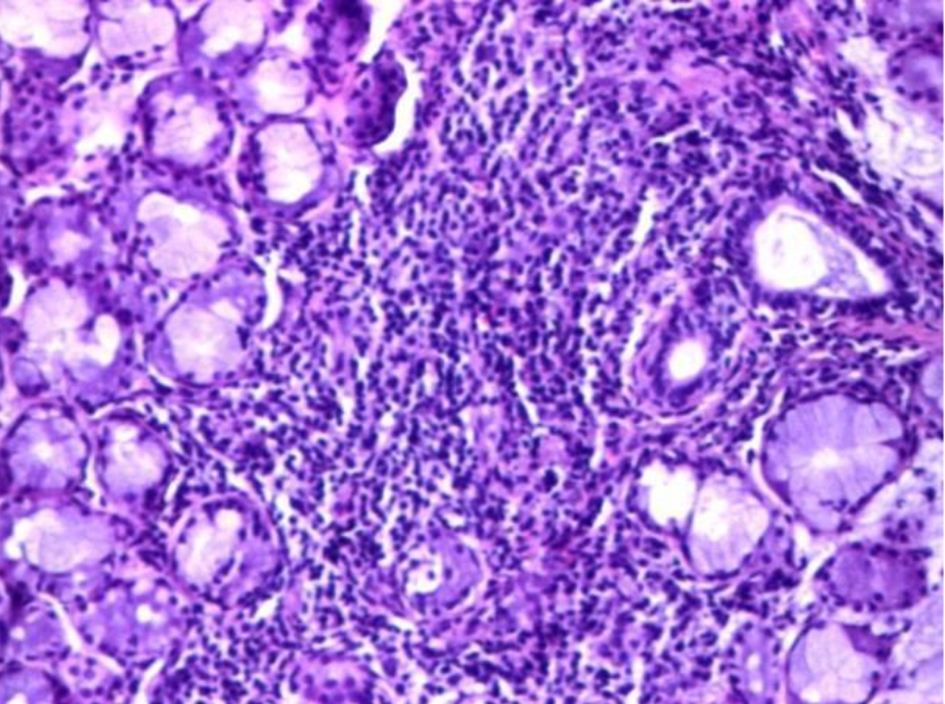 Click for large image | Figure 2. The accessory salivary gland biopsy showing grade 4 lymphocytic sialadenitis from Chisholm and Mason (hematoxylin and eosin, magnification × 10). |
| Discussion | ▴Top |
The collecting tube is the site of final regulation of urinary acid excretion, and the type A intercalated cells in this segment perform the function of distal H+ ion secretion and HCO3 reabsorption. The secretion of H+ is effected by the H+-ATPase and H+, K+-ATPase pumps. The H+-ATPase pump is abundantly expressed in the membrane and in the cytoplasmic vesicles of the apical pole of type A intercalated cells. The proton secretion activity is coupled with HCO3 reabsorption activity carried out by the renal isoform of the basolateral Cl-/HCO3 exchanger also known as AE1. The Cl- ion which enters the cell through the activity of the AE1 exchanger is recycled via the basolateral K+-Cl- cotransporter or the Cl- ClC-kb channel. Defective type A intercalated cells function will result in hyperchloremic acidosis with urinary pH unsuitable for acidosis (> 5.5) and insufficient net acid excretion. In normal conditions, alkaline urine (pH > 7.6) stimulates the secretion of H+ and this gradient is greater than 20 mm Hg. Hyperchloremic acidosis is associated with hypokalemia, hypercalciuria, bone disease (rickets or osteomalacia) and hypocitraturia. Hypokalemia is explained by stimulation of distal potassium secretion by unreabsorbed bicarbonate and stimulation of the renin-angiotensin-aldosterone system secondary to sodium loss. Chronic acidosis stimulates proximal reabsorption of citrate (one molecule of citrate produces three molecules of bicarbonate) and bone resorption; hypocitraturia, alkaline urine and hypercalciuria will lead to nephrocalcinosis and/or nephrolithiasis [2]. The pathogenesis of renal involvement and the pathophysiology of DTA1 during SS are still unclear [3], and probably multifactorial [4].
Kidney disease can be the consequence of tubulointerstitial infiltration of T cells, B cells, plasma cells and, more rarely, of autoantibodies [5, 6]. The majority of patients exhibit clinical manifestations that are a direct consequence of interstitial infiltration of lymphocytes, which promotes interstitial fibrosis leading to chronic kidney disease. Tubulitis has also been associated with distal DTA and leads to the complete absence of H+-ATPase in the collecting ducts [6, 7] and thiazide-sensitive NaCl cotransporter (NCCT) [5]. Autoantibodies have also been found against NCCT [5], although their precise role in eliciting clinical symptoms remains to be established. Autoantibodies to carbonic anhydrase were found in the serum of patients with lupus erythematosus systemic and SS and were also detected in the renal distal tubules [8]. Autoantibodies to carbonic anhydrase enzymes have been identified in the serum of patients with primary SS [9] and seem to correlate with DTA. Whether these autoantibodies are a consequence of, or contribute to renal injury is not clear, although the induction of antibodies to carbonic anhydrase in mice can reproduce DTA [10]. Some of these autoantibody targets (e.g., carbonic anhydrase II and H+-ATPase) can be found in the salivary glands and in renal intercalated cells [11]. Salivary gland injury, therefore, might cause a release of autoantigens in the kidney, which consequently become the target of both T lymphocytes and autoantibodies. Moreover, several studies have found a positive correlation between disease duration [12], hypergammaglobulinemia and the presence of autoantibodies against SSA (Ro) or SSB (La) [13].
The diagnostic delay that is observed in our patient is due to the fact that clinicians are not used to further exploration for hypokalemia and the absence of evident extra-renal signs of SS. The clinical manifestation is also characterized by repetitive episodes of hypokalemic crisis with paralysis of the limbs and which often constitutes a differential diagnosis with Westphal’s disease and thyrotoxic hypokalemic paralysis.
It is clear that corticosteroid therapy is not effective on lesions of chronic tubulointerstitial nephropathy. However, DTA1 is mainly attributed at the etiopathogenetic level to immunological phenomena (see above) and not to organic tubulointerstitial involvement. So it makes sense to use corticosteroids during DTA1. Study of the literature shows that corticosteroid therapy has been used in several reported cases but the effectiveness is not established yet [1].
Conclusion
The pathogenesis of renal involvement and the pathophysiology of DTA1 during SS appear to be related to immunological factors. The use of corticosteroids may be necessary in the management.
Acknowledgments
None to declare.
Financial Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent for publication was obtained from the patient.
Author Contributions
Mansour Mbengue reviewed the literature and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript. Cedric Ouanekpone revised the article critically for important intellectual content. Seynabou Diagne revised the article critically for important intellectual content and gave final approval of the version to be submitted. Abdou Niang revised the article critically for important intellectual content and gave final approval of the version to be submitted.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Francois H, Mariette X. Renal involvement in primary Sjogren syndrome. Nat Rev Nephrol. 2016;12(2):82-93.
doi pubmed - Traite de nephrologie. Thervet E, directeurs. Traite de nephrologie. Paris: Lavoisier; 2017.
- Karras D, Antoniadis C, Papadakis J, Vafiadis S. [Acute flaccid quadriplegia disclosing Gougerot-Sjogren syndrome with renal tubular acidosis, nephrocalcinosis and osteomalacia]. Rev Rhum Mal Osteoartic. 1989;56(6):491-494.
- Aerts J, Vigouroux C, Fournier P, Cariou D, Pasquier P. [Osteomalacia of renal origin disclosing Gougerot-Sjogren syndrome]. Rev Med Interne. 1994;15(1):43-47.
doi - Kim YK, Song HC, Kim WY, Yoon HE, Choi YJ, Ki CS, Park CW, et al. Acquired Gitelman syndrome in a patient with primary Sjogren syndrome. Am J Kidney Dis. 2008;52(6):1163-1167.
doi pubmed - Cohen EP, Bastani B, Cohen MR, Kolner S, Hemken P, Gluck SL. Absence of H(+)-ATPase in cortical collecting tubules of a patient with Sjogren's syndrome and distal renal tubular acidosis. J Am Soc Nephrol. 1992;3(2):264-271.
- DeFranco PE, Haragsim L, Schmitz PG, Bastani B. Absence of vacuolar H(+)-ATPase pump in the collecting duct of a patient with hypokalemic distal renal tubular acidosis and Sjogren's syndrome. J Am Soc Nephrol. 1995;6(2):295-301.
- Inagaki Y, Jinno-Yoshida Y, Hamasaki Y, Ueki H. A novel autoantibody reactive with carbonic anhydrase in sera from patients with systemic lupus erythematosus and Sjogren's syndrome. J Dermatol Sci. 1991;2(3):147-154.
doi - Pertovaara M, Bootorabi F, Kuuslahti M, Pasternack A, Parkkila S. Novel carbonic anhydrase autoantibodies and renal manifestations in patients with primary Sjogren's syndrome. Rheumatology (Oxford). 2011;50(8):1453-1457.
doi pubmed - Takemoto F, Katori H, Sawa N, Hoshino J, Suwabe T, Sogawa Y, Nomura K, et al. Induction of anti-carbonic-anhydrase-II antibody causes renal tubular acidosis in a mouse model of Sjogren's syndrome. Nephron Physiol. 2007;106(4):p63-68.
doi pubmed - Kim J, Tisher CC, Linser PJ, Madsen KM. Ultrastructural localization of carbonic anhydrase II in subpopulations of intercalated cells of the rat kidney. J Am Soc Nephrol. 1990;1(3):245-256.
- Pertovaara M, Korpela M, Kouri T, Pasternack A. The occurrence of renal involvement in primary Sjogren's syndrome: a study of 78 patients. Rheumatology (Oxford). 1999;38(11):1113-1120.
doi pubmed - Aasarod K, Haga HJ, Berg KJ, Hammerstrom J, Jorstad S. Renal involvement in primary Sjogren's syndrome. QJM. 2000;93(5):297-304.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
