| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Original Article
Volume 11, Number 1, June 2022, pages 18-23
Efficacy of Top Flat Magnetic Stimulation Technology for Female Stress and Urge Urinary Incontinence: A Clinical Evaluation
Andrea Biondoa, Pablo Gonzalez Isazab, Irene Fuscoc, d
aDr. Andrea Biondo’s Clinic, Palermo, Italy
bHospital Universitario San Jorge and Liga Contra el Cancer, Pereira, Colombia
cDepartment of Pharmacology, University of Florence, Florence, Italy
dCorresponding Author: Irene Fusco, Department of Pharmacology, University of Florence, Florence, Italy
Manuscript submitted April 20, 2022, accepted May 26, 2022, published online June 16, 2022
Short title: Top Flat Magnetic Stimulation
doi: https://doi.org/10.14740/wjnu432
| Abstract | ▴Top |
Background: Urinary incontinence (UI) is a popular problem with a broad range of severities and etiology. This study evaluates the effectiveness and safety of a device based on top flat magnetic stimulation to manage women affected by urge urinary incontinence (UUI) and stress urinary incontinence (SUI).
Methods: Eighty-one female patients (35 patients who reported SUI symptoms and 46 patients who reported UUI symptoms) underwent a total of eight treatments sessions performed twice a week for 4 consecutive weeks, for 28 min. Immediately before each treatment and up to 3 months of follow-up, two questionnaires (Incontinence Impact Questionnaire-Short Form (IIQ-7) and Incontinence Questionnaire Overactive Bladder Module (ICIQ-OAB)) were used.
Results: ICIQ-OAB’s average score significantly decreased (P < 0.05) from 10.54 ± 3.03 at baseline to 5.45 ± 2.24 at 3 months follow-up. IIQ-7’s average score significantly decreased (P < 0.05) from 15.53 ± 5.62 at baseline to 6.76 ± 3.10 at 3 months follow-up.
Conclusions: For all women examined, protocols used led to a decrease of SUI/UUI complaints, achieving good results and improving patients’ quality of life (QOL) without risk.
Keywords: Urge urinary incontinence; Stress urinary incontinence; Quality of life; Top flat magnetic stimulation technology
| Introduction | ▴Top |
Urinary incontinence (UI) is a popular condition with symptoms of varying nature and severity and can affect women of all ages. UI can seriously affect the psychological, physical, and social welfare of people. The principal types are mixed and stress UI.
The overactive bladder (OAB) syndrome is described as urgency which develops ordinarily with nocturia, with or without urge UI [1]. Following the observation of involuntary bladder contractions urodynamic studies, OAB can coexist with detrusor overactivity (DO) in both males and females.
Stress urinary incontinence (SUI) derives from weak and supportive urethral muscles. Urge urinary incontinence (UUI) results from OAB muscle, and mixed when both symptoms may coexist.
To establish a diagnosis and exclude a urinary tract infection, physical tests, such as a stress test of the vaginal exam and measurement of residual postvoid volume, are performed. In this perspective, the current study shed light on evaluating the effectiveness and safety of a device that uses top flat magnetic stimulation to manage women who reported SUI/UUI symptoms.
| Materials and Methods | ▴Top |
Patients
The patient’s UI status was evaluated by a gynecologist. Participants were graded as SUI or UUI patients based on pertinent questions of the questionnaires chosen for the study concerning inclusive criteria for all types of UI, in conformity with the UI classification of the International Continence Society [2].
A total of 81 patients were included for this study. Subjects were divided in two groups: 35 female patients (average age of 45.61 ± 10.39 years) who met the criteria for SUI were classified as group A, and 46 female patients (average age of 58.36 ± 14,86) who met the criteria for UUI were classified as group B. Demographic features of patients are listed in Table 1.
 Click to view | Table 1. Demographic Characteristics of Patients |
Exclusion criteria included: pelvic organ prolapse beyond the hymen established using International Continence Society Pelvic Organ Prolapse Quantification (POP-Q) system [3]; active infection of the urinary tract or with human papillomavirus (HPV) or herpes; use of vaginal estrogen therapy in the prior 6 months or diuretics; abnormal vaginal bleeding; previous surgical SUI treatment or pelvic radiotherapy; patients with implanted defibrillators/neurostimulators, cardiac pacemakers, electronic and metal implantations, heart disorders, pulmonary insufficiency, malignant tumor, severe neurological diseases, obesity and pregnancy.
Study device
DR. ARNOLD (DEKA MELA Calenzano, Italy) is a noninvasive therapeutic device for the treatment of UI which selectively lead to a stimulation of the female pelvic floor muscles (PFMs) with an electromagnetic field characterized by a homogeneous profile (TOP FMS - TOP Flat Magnetic Stimulation). With this type of stimulation, effects on blood circulatory system, muscle contraction, and depolarization of neuronal cells are obtained.
This greater homogeneity of distribution of the magnetic field in a wider area that allows greater recruitment of muscle fibers without producing zones of varying intensity of stimulation represents one of the great advantages of our device.
The subject system is a CE-marked device since July 2020. The subject device has a central unit and a chair applicator planned for deep pelvic floor area therapy, as shown in Figure 1.
 Click for large image | Figure 1. Representation of DR. ARNOLD’s chair. Courtesy of DEKA MELA company. |
At the initial stages of treatment, before each session, a gynecologist or a trained operator set and adjusted the patient’s position; patient’s perineum is positioned in the center of the seat where the coil is placed to ensure a proper intensity of stimulation and to achieve a muscle contractions uniform distribution.
With the use of subject device, the muscles work at the same intensity in all the area considered. A trained assistant or a physician set stimulation intensity and patient’s chair position before each treatment to consent an adequate stimulation and to ensures patient comfort during the treatment. For a precise position of the patient before therapy, the seat height has been adjusted, so that the patient’s legs are perpendicularly flexed, the thighs are parallel to the floor and the feet are flat on the ground.
Assessments and study protocol
All patients underwent eight treatments with the DR. ARNOLD system (DEKA MELA Calenzano, Italy). Sessions were performed twice a week for 4 straight weeks, for 28 min.
All patients started with a short warm-up phase; patients of group A underwent four sessions with the protocol Hypotonus/Weakness 1 and four sessions of the protocol Hypotonus/Weakness 2 [4]. Patients of group B underwent eight sessions with the protocol Overtone/Pain protocol (muscle work aimed at muscle inhibition). The patient’s position and intensity of the stimulation were set before each treatment to ensure adequate stimulation.
Two questionnaires were used to evaluate the urinary improvements: Incontinence Questionnaire Overactive Bladder Module (ICIQ-OAB) [5] has been assigned and filled out by patients of group B, and Incontinence Impact Questionnaire-Short Form (IIQ-7) [6] has been assigned and filled out by patients of group A. The two questionnaires were completed before each treatment and up to 3 months follow-up (3MFU).
A high score shows an important presence of symptoms, while the decrease indicates improvement. Side effects, including tendon pain, muscular pain, local erythema, skin redness and temporary muscle spasm, were evaluated during the entire treatment periods.
Institutional Review Board approval is not necessary as DR. ARNOLD system is a CE-marked device since July 2020. The study was conducted in accordance with the Declaration of Helsinki.
Statistics
Student’s t-test and SPSS (IBM Corp., New York, USA) were performed. Data were shown as means ± standard deviation (SD).
| Results | ▴Top |
According to questionnaire results, both improvements in UUI and SUI symptoms were observed, at baseline and after treatment session at 3MFU, as shown in Table 2.
 Click to view | Table 2. IIQ-7 and ICIQ-OAB Questionnaire Mean Score (± SD) Evaluated for Group A and Group B at Baseline and up to 3 Months Follow-Up |
All two questionnaire mean scores significantly decreased (P < 0.05).
ICIQ-OAB’s average score significantly decreased (P < 0.05) from 10.54 ± 3.03 at baseline to 5.45 ± 2.24 at 3MFU. IIQ-7’s average score significantly decreased (P < 0.05) from 15.53 ± 5.62 at baseline to 6.76 ± 3.10 at 3MFU (Figs. 2, 3).
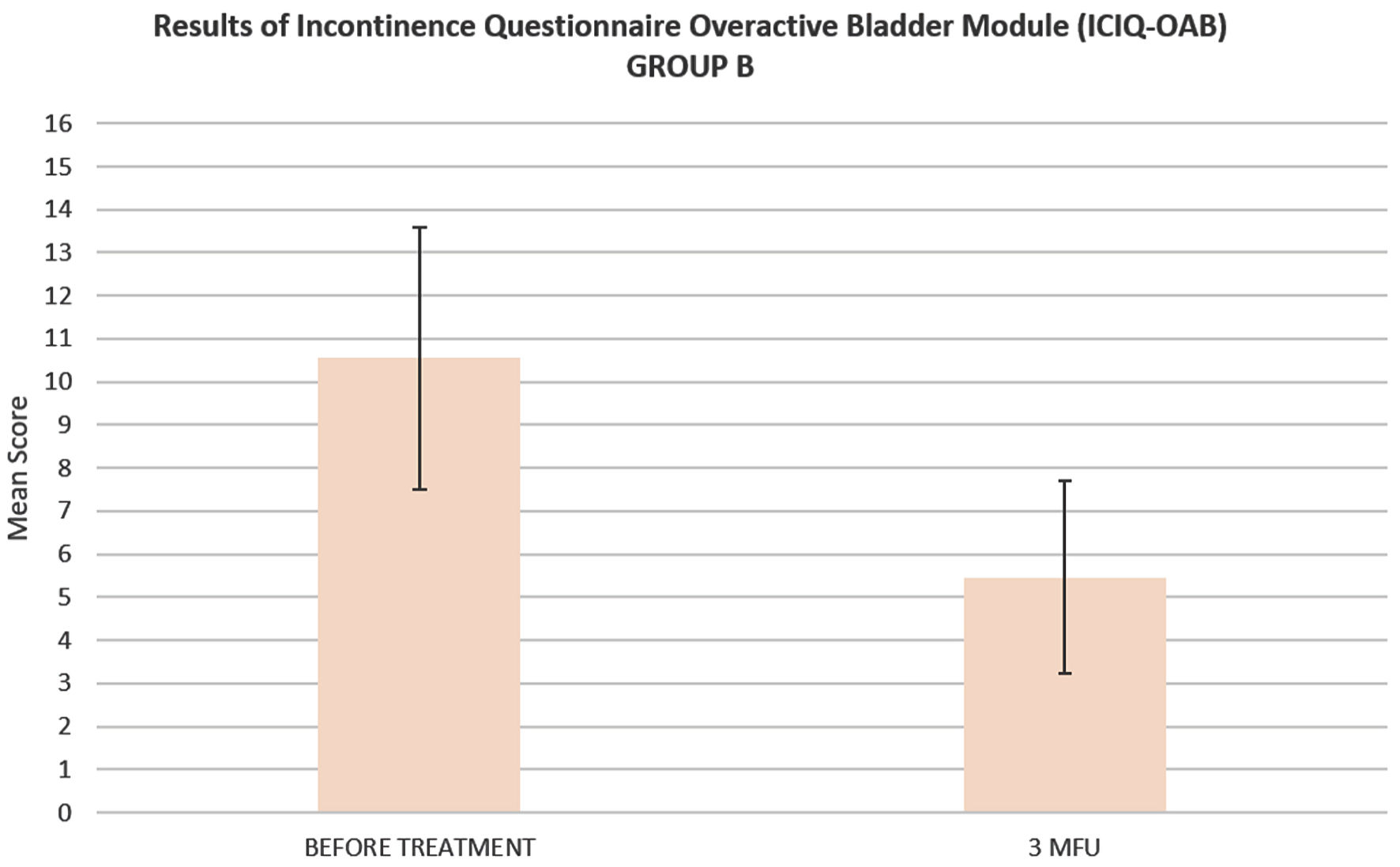 Click for large image | Figure 2. Histogram representation of ICIQ-OAB mean score of patients suffering from UUI (group B), before the treatment and up to 3 months follow-up. ICIQ-OAB: Incontinence Questionnaire Overactive Bladder Module; UUI: urge urinary incontinence; 3MFU: 3 months follow-up. |
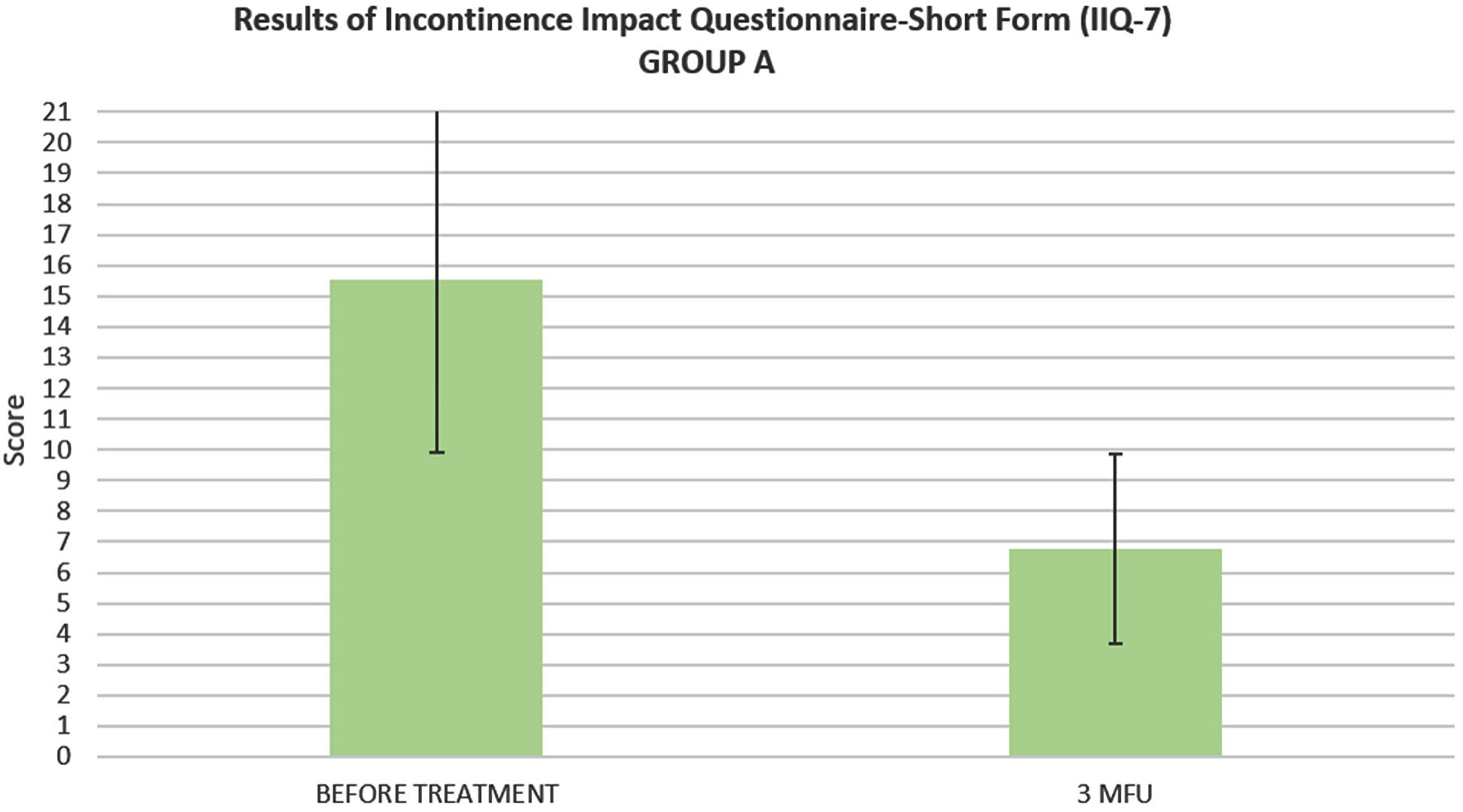 Click for large image | Figure 3. Histogram representation of IIQ-7 mean score of patients suffering from SUI (group A), before the treatment and up to 3 months follow-up. IIQ-7: Incontinence Impact Questionnaire-Short Form; SUI: stress urinary incontinence; 3MFU: 3 months follow-up. |
No side effects were reported.
| Discussion | ▴Top |
Pharmacological treatment of SUI is restricted. The only pharmacological options available are the serotonin and norepinephrine reuptake inhibitors [7], whose common adverse effects were report in several clinical studies, such as constipation, dry mouth, nausea, fatigue, migraine, insomnia, and diarrhea [8]. For OAB or UUI, drugs such as the newly agonist of beta-3-adrenoreceptor mirabegron, or antimuscarinics, are well established. However, they may be poorly tolerated [9], and side effects such as constipation, blurred vision, and a reduction of salivation were observed.
When antimuscarinic treatments fail to alleviate symptoms, patients might require invasive treatments, such as botulinum neurotoxin-A (BoNT-A) injections, posterior tibial nerve or central neuromodulation [10].
Other therapy for SUI/UUI consists of conservative measures. This includes the use of mid-urethral slings, modification of fluid intake [11] or avoiding caffeinated drinks, electrical stimulation (ES), weight loss, and pelvic floor training (Kegel exercises); but patients should be encouraged to persevere with these treatments [12]. Since Kegel exercises are often not performed gradually and correctly by patients, their effectiveness is reduced [13].
Several studies demonstrated the efficacy of magnetic stimulation (MS) in the management of SUI/UUI conditions [14-19] and in mixed urinary incontinence (MUI) patients [20, 21]. In comparison to ES, MS requires that less current be generated on the body surface. Thus, MS, by inducing electric currents, can activate deep neural structures without discomfort or pain for the patient. Furthermore, as a result of the deep penetration of the electromagnetic field in the pelvic area, a greater activation of the muscle force of the PFM is observed compared to electro-stimulators, with which most of the energy emitted is dispersed on the surface. MS treatment leads to an improvement in patient’s UI symptoms and quality of life (QOL), with no side effects reported. However, the longer-term treatment outcomes must be determined by long-term trials [22]. Recently published studies [4, 23] already demonstrated the efficacy of top flat magnetic stimulation both in the management of physical condition in female subjects and male UI after radical prostatectomy with good and promising results.
Although incontinence is not a life-threatening condition it can substantially affect the QOL. Several studies are examining the effects of SUI and UUI on QOL [24]. An anxiety and depression symptoms increasing in patients with UI, as well as a degeneration of general QOL, were observed. People with UI may experience severe limitation and embarrassment in their daily activities such as going out or exercising. UI can also affect women relationships due to the risk of leak during the various steps of intercourse.
For the majority of subjects, conservative treatments are disposable for these main kinds of UI, but there are significant limitations [25].
The magnetic stimulator technology works by exerting a deep stimulation of the PFMs and an efficient recovery of neuromuscular control in these patients [26]. According to the scientific literature, this technology, with a PFM strengthening, can safely and effectively treat urge, stress and mixed UI across a broad demographic of patients, as discussed above. This innovative technology triggers intense contractions of the PFMs, inducing electrical currents which depolarized neurons, causing concentric contractions, and lifting the entire PFM [18]. The key efficacy of the subject device is based, thanks to electromagnetic energy, on a neuromuscular control recovery and a deep PFM stimulation.
Our findings clearly show benefits of top flat magnetic stimulation in both SUI and UUI conditions. According to questionnaire results, our findings indicate an improvement in UUI and SUI symptoms at baseline and after treatment session at 3MFU, showing a significantly reduction of both ICIQ-OAB and IIQ-7 mean scores of treated subjects. Patients reported a reduction of UI symptoms severity, which has had a positive impact on their QOL. Based on the qualitative assessment, patients also reported a better control of urination and increased sexual satisfaction.
In comparison to the other treatment modalities for pelvic floor restoration, this technology has significant benefits. To stimulate the muscles, it does not need the use of a probe and thanks to the regular emission of progressively supplied energy, it allows patients to remain fully clothed in a comfortable and ergonomic seat. Patients can perceive the relaxation of the muscles involved in the treatment, making them acquire greater self-awareness and recover their daily activities immediately after treatments.
Furthermore, the possibility that the device is be able to use different types of protocols makes it effective to treat different types of pathological conditions linked to UI. Finally, the subject device can also be used in combination with other pharmacological or physical methods [27].
Study limitations
Limitation of the current study includes the lack of a control group and of urodynamic testing to establish a diagnosis of DO. Our future goal will be to implement these aspects with further investigation.
Conclusions
Based on the study results, this technology could represent a new treatment option for SUI/UUI conditions. For all women examined, the protocols used led to a reduction in SUI/UUI symptoms being minimally invasive and improving patients’ QOL without risk.
Acknowledgments
None to declare.
Financial Disclosure
No external funding was provided.
Conflict of Interest
No conflict of interest to declare.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Author Contributions
Conceptualization, AB; methodology, AB; software, AB; validation, AB and PGI; formal analysis, AB; investigation, PGI and AB; resources, AB; data curation, IF and AB; writing-original draft preparation, IF; writing-review and editing, IF, PGI and AB; visualization, AB, IF and PGI; supervision, AB, PGI and IF; project administration, AB; funding acquisition, AB. All authors have read and agreed to the published version of the manuscript.
Data Availability
Data that support the study findings are available on request from the corresponding author.
| References | ▴Top |
- Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary incontinence in women: a review. JAMA. 2017;318(16):1592-1604.
doi pubmed - Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5-26.
doi pubmed - Persu C, Chapple CR, Cauni V, Gutue S, Geavlete P. Pelvic Organ Prolapse Quantification System (POP-Q) - a new era in pelvic prolapse staging. J Med Life. 2011;4(1):75-81.
- Lopopolo G, Salsi B, Banfi A, Isaza PG, Fusco I. Is it possible to improve urinary incontinence and quality of life in female patients? A clinical evaluation of the efficacy of top flat magnetic stimulation technology. Bioengineering (Basel). 2022;9(4):140.
doi pubmed - Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77(6):805-812.
doi pubmed - Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14(2):131-139.
doi pubmed - Dmochowski RR, Miklos JR, Norton PA, Zinner NR, Yalcin I, Bump RC, Duloxetine Urinary Incontinence Study G. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170(4 Pt 1):1259-1263.
doi pubmed - Bump R, et al. The effect of duloxetine dose escalation and tapering on the incidence of adverse events (AE) in women with stress urinary incontinence (SUI). Neurourol Urodyn. 2005;24:582-583.
- Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326(7394):841-844.
doi pubmed - Humburg J. [Female urinary incontinence: diagnosis and treatment]. Ther Umsch. 2019;73(9):535-540.
doi pubmed - Hashim H, Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008;102(1):62-66.
doi pubmed - Shaban A, Drake MJ, Hashim H. The medical management of urinary incontinence. Auton Neurosci. 2010;152(1-2):4-10.
doi pubmed - Yount SM, Fay RA, Kissler KJ. Prenatal and postpartum experience, knowledge and engagement with Kegels: a longitudinal, prospective, multisite study. J Womens Health (Larchmt). 2021;30(6):891-901.
doi pubmed - Galloway NT, El-Galley RE, Sand PK, Appell RA, Russell HW, Carlan SJ. Extracorporeal magnetic innervation therapy for stress urinary incontinence. Urology. 1999;53(6):1108-1111.
doi - Galloway NT, El-Galley RE, Sand PK, Appell RA, Russell HW, Carlin SJ. Update on extracorporeal magnetic innervation (EXMI) therapy for stress urinary incontinence. Urology. 2000;56(6 Suppl 1):82-86.
doi - Gilling PJ, Wilson LC, Westenberg AM, McAllister WJ, Kennett KM, Frampton CM, Bell DF, et al. A double-blind randomized controlled trial of electromagnetic stimulation of the pelvic floor vs sham therapy in the treatment of women with stress urinary incontinence. BJU Int. 2009;103(10):1386-1390.
doi pubmed - Samuels J, Guerette N. Hifem technology - the non-invasive treatment of urinary incontinence. 38th American society for laser medicine and surgery annual conference on "energy-based medicine and science". 2018;50:11-15.
- Vadala M, Palmieri B, Malagoli A, Laurino C. High-power magnetotherapy: a new weapon in urinary incontinence? Low Urin Tract Symptoms. 2018;10(3):266-270.
doi pubmed - Elena S, Dragana Z, Ramina S, Evgeniia A, Orazov M. Electromyographic evaluation of the pelvic muscles activity after high-intensity focused electromagnetic procedure and electrical stimulation in women with pelvic floor dysfunction. Sex Med. 2020;8(2):282-289.
doi pubmed - Samuels JB, Pezzella A, Berenholz J, Alinsod R. Safety and efficacy of a non-invasive High-Intensity Focused Electromagnetic Field (HIFEM) device for treatment of urinary incontinence and enhancement of quality of life. Lasers Surg Med. 2019;51(9):760-766.
doi pubmed - Cidranes D, Blanco E. Safety and preliminary efficacy of magnetic stimulation of pelvic floor with hifem technology in urinary incontinence. Medical & Clinical Research. 2018;3(2):2577-8005.
doi - Gozlersuzer O, Yalvac B, Cakiroglu B. Investigation of the effectiveness of magnetic field therapy in women with urinary incontinence: Literature review. Urologia. 2022.
doi pubmed - Isaza PG, Borrego RS, Fusco I. A case of stress urinary incontinence after radical prostatectomy successfully treated with an innovative device based on top flat magnetic stimulation. World J Urol. 2022.
doi pubmed - Coyne KS, Zhou Z, Thompson C, Versi E. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92(7):731-735.
doi pubmed - Yip SK, Chan A, Pang S, Leung P, Tang C, Shek D, Chung T. The impact of urodynamic stress incontinence and detrusor overactivity on marital relationship and sexual function. Am J Obstet Gynecol. 2003;188(5):1244-1248.
doi pubmed - Yamanishi T, Suzuki T, Sato R, Kaga K, Kaga M, Fuse M. Effects of magnetic stimulation on urodynamic stress incontinence refractory to pelvic floor muscle training in a randomized sham-controlled study. Low Urin Tract Symptoms. 2019;11(1):61-65.
doi pubmed - Rosen NO, Dawson SJ, Brooks M, Kellogg-Spadt S. Treatment of vulvodynia: pharmacological and non-pharmacological approaches. Drugs. 2019;79(5):483-493.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
